
Fluorocarbon
Encyclopedia
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
bonded together in strong carbon–fluorine bond
Carbon–fluorine bond
The carbon–fluorine bond is a bond between carbon and fluorine that is a component of all organofluorine compounds. It is the strongest single bond in organic chemistry—and relatively short—due to its partial ionic character. The bond also strengthens and shortens as more fluorines are...
s. Fluoroalkanes that contain only single bonds are more chemically and thermally stable than alkanes. However, fluorocarbons with double bonds (fluoroalkenes) and especially triple bonds (fluoroalkynes) are more reactive than their corresponding hydrocarbons. Fluoroalkanes can serve as oil-repellent
Lipophobicity
Lipophobicity, also sometimes called lipophobia, is a chemical property of chemical compounds which means "fat rejection", literally "fear of fat". Lipophobic compounds are those not soluble in lipids or other non-polar solvents...
/water-repellent
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
fluoropolymers, solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s, liquid breathing research
Liquid breathing
Liquid breathing is a form of respiration in which a normally air-breathing organism breathes an oxygen-rich liquid , rather than breathing air....
agents, and powerful greenhouse gases. Unsaturated
Unsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
fluorocarbons tend to be used as reactants.
Many chemical compounds are labeled as fluorocarbons, perfluorinated, or with the prefix perfluoro- despite containing atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s other than carbon or fluorine, such as chlorofluorocarbons and perfluorinated compounds
Perfluorinated compounds
A perfluorinated compound is an organofluorine compound with all hydrogens replaced by fluorine on a carbon chain—but the molecule also contains at least one different atom or functional group. Thus, PFCs have properties similar to fluorocarbons as they are fluorocarbon derivatives...
; however, these molecules are fluorocarbon derivatives, and not true fluorocarbons. Fluorocarbon derivatives share many of the properties of fluorocarbons, while also possessing new properties due to the inclusion of new atoms. For example, fluorocarbon derivatives can function as fluoropolymer
Fluoropolymer
A fluoropolymer is a fluorocarbon based polymer with multiple strong carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases.-History:Fluoropolymers were accidentally discovered in 1938 by Dr. Roy J...
s, refrigerants, solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s, anesthetics, fluorosurfactant
Fluorosurfactant
Fluorosurfactants, or fluorinated surfactants, are synthetic organofluorine chemical compounds that have multiple fluorine atoms. They can be polyfluorinated or fluorocarbon-based . As surfactants, they are more effective at lowering the surface tension of water than comparable hydrocarbon...
s, and ozone depletors
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
.
Usage of term
The formal IUPAC definition of a fluorocarbon is a molecule consisting wholly of fluorine and carbon. However, other fluorocarbon based molecules that are not technically fluorocarbons are commonly referred to as fluorocarbons, because of similar structures and identical properties. Compounds with atoms other than carbon and fluorine are not true fluorocarbons and they are considered as fluorocarbon derivatives in a separate section below.Physical properties
Fluorocarbon liquids are colorless. They have high density, up to over twice that of water, due to their high molecular weight. Low intermolecular forceIntermolecular force
Intermolecular forces are forces of attraction or repulsion which act between neighboring particles: atoms, molecules or ions. They are weak compared to the intramolecular forces, the forces which keep a molecule together...
s give the liquids low viscosities when compared to liquids of similar boiling points. Also, low surface tension, heats of vaporization, and refractive indices
Refractive index
In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
are notable. They are not miscible with most organic solvents (e.g., ethanol, acetone, ethyl acetate and chloroform), but are miscible with some hydrocarbons (e.g., hexane in some cases). They have very low solubility in water, and water has a very low solubility in them (on the order of 10 ppm). The number of carbon atoms in a fluorocarbon molecule largely determines most physical properties. The greater the number of carbon atoms, the higher the boiling point, density, viscosity, surface tension, critical properties, vapor pressure and refractive index. Gas solubility decreases as carbon atoms increase, while melting point is determined by other factors as well and is thus not readily predicted.
London dispersion force reduction
As the high electronegativityElectronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of fluorine reduces the polarizability
Polarizability
Polarizability is the measure of the change in a molecule's electron distribution in response to an applied electric field, which can also be induced by electric interactions with solvents or ionic reagents. It is a property of matter...
of the atom, fluorocarbons are only weakly susceptible to the fleeting dipoles that form the basis of the London dispersion force
London dispersion force
London dispersion forces is a type of force acting between atoms and molecules. They are part of the van der Waals forces...
. As a result, fluorocarbons have low intramolecular attractive forces and are lipophobic in addition to being hydrophobic/non-polar. Thus fluorocarbons find applications as oil-, water-, and stain-repellents in products such as Gore-Tex
Gore-Tex
Gore-Tex is a waterproof/breathable fabric, and a registered trademark of W. L. Gore and Associates. It was co-invented by Wilbert L. Gore, Rowena Taylor, and Gore's son, Robert W. Gore. Robert Gore was granted on April 27, 1976, for a porous form of polytetrafluoroethylene with a...
and fluoropolymer carpet coatings. The reduced participation in the London dispersion force makes the solid polytetrafluoroethylene
Polytetrafluoroethylene
Polytetrafluoroethylene is a synthetic fluoropolymer of tetrafluoroethylene that finds numerous applications. PTFE is most well known by the DuPont brand name Teflon....
(PTFE) slippery as it has a very low coefficient of friction. Also, the low attractive forces in fluorocarbon liquids make them compressible and gas soluble while smaller fluorocarbons are extremely volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
. There are five fluoroalkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
gases; tetrafluoromethane
Tetrafluoromethane
Tetrafluoromethane, also known as carbon tetrafluoride, is the simplest fluorocarbon . It has a very high bond strength due to the nature of the carbon–fluorine bond. It can also be classified as a haloalkane or halomethane...
(bp −128 °C), hexafluoroethane
Hexafluoroethane
Hexafluoroethane is a fluorocarbon counterpart to the hydrocarbon ethane. It is a non-flammable gas negligibly soluble in water and slightly soluble in alcohol.-Physical properties:...
(bp −78.2 °C), octafluoropropane
Octafluoropropane
Octafluoropropane is a fluorocarbon non-flammable greenhouse gas that can be produced either by electrochemical fluorination or by the Fowler process using cobalt fluoride.-Applications:...
(bp −36.5 °C), perfluoro-n-butane
Perfluorobutane
Perfluorobutane is a colorless gas. It is a simple fluorocarbon with the n-butane skeleton, but with hydrogen atoms in n-butane are replaced with fluorine atoms. It is used as a replacement for Halon 1301 fire extinguishers, as well as being an ultrasound imaging agent. As a pharmaceutical, it is...
(bp −2.2 °C) and perfluoro-iso-butane (bp −1 °C). Nearly all other fluoroalkanes are liquids with the exception of perfluorocyclohexane, which sublimes at 51 °C. As a result of the high gas solubility of fluorocarbon liquids, they have been the subject of medical research as blood carriers because of their oxygen solubility. Fluorocarbons also have low surface energies
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
and high dielectric
Dielectric
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric is placed in an electric field, electric charges do not flow through the material, as in a conductor, but only slightly shift from their average equilibrium positions causing dielectric...
strengths.

Fluoroalkane stability
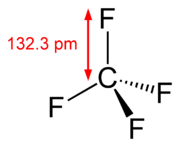
Fluorocarbons with only single bonds are very stable because of the strength and nature of the carbon–fluorine bond. It is called the strongest bond in organic chemistry. Its strength is a result of the electronegativity of fluorine imparting partial ionic character through partial chargePartial charge
A partial charge is a charge with an absolute value of less than one elementary charge unit .-Partial atomic charges:...
s on the carbon and fluorine atoms. The partial charges shorten and strengthen the bond through favorable coulombic interactions. Additionally, multiple carbon–fluorine bonds increase the strength and stability of other nearby carbon–fluorine bonds on the same geminal
Geminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
carbon, as the carbon has a higher positive partial charge. Furthermore, multiple carbon–fluorine bonds also strengthen the "skeletal" carbon–carbon bonds from the inductive effect
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
. Therefore, saturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
fluorocarbons are more chemically and thermally stable than their corresponding hydrocarbon counterparts. However, fluoroalkanes are not inert
Inert
-Chemistry:In chemistry, the term inert is used to describe a substance that is not chemically reactive.The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions...
. They are susceptible to reduction through the Birch reduction
Birch reduction
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
.
Fluoroalkene and fluoroalkyne reactivity
When fluorocarbons are unsaturatedUnsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
, they are less stable and more reactive than fluoroalkanes, or comparable hydrocarbons, due to the electronegativity of fluorine. The reactivity of the simplest fluoroalkyne, difluoroacetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
, is an example of this instability; difluoroacetylene easily polymerizes. Another example is fluorofullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
, which has weaker and longer carbon–fluorine bonds than saturated fluorocarbons. It is reactive towards nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s and hydrolyzes
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
in solution. Additionally, the polymerization of the fluoroalkene tetrafluoroethylene
Tetrafluoroethylene
Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:...
(which results in PTFE) is more energetically favorable than that of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
. Unsaturated fluorocarbons have a driving force towards sp3 hybridization due to the electronegative fluorine atoms seeking a greater share of bonding electrons with reduced s character in orbitals.
One notable exception to this trend is fluorobenzene
Fluorobenzene
Fluorobenzene is the chemical compound with the formula C6H5F, often abbreviated PhF. This species is a derivative of benzene, with a single fluorine atom attached. Its melting point is 44 °C lower than that of benzene, indicative of the remarkable effect of fluorination on the intermolecular...
, which is stabilized by its aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
.
Manufacture
Prior to World War IIWorld War II
World War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
, the only known route to fluorocarbons was by direct reaction of fluorine with the hydrocarbon. This highly exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
process was capable only of synthesising tetrafluoromethane, hexafluoroethane and octafluoropropane; larger hydrocarbons decomposed in the extreme conditions. The Manhattan project
Manhattan Project
The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army...
saw the need for some very robust chemicals, including a wider range of fluorocarbons, requiring new manufacturing methods. The so-called "catalytic" method involved reacting fluorine and hydrocarbon on a bed of gold-plated copper turnings, the metal removing the heat of the reaction (so not really acting as a catalyst at all), allowing larger hydrocarbons to survive the process. However, it was the Fowler process
Fowler process
The Fowler Process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt fluoride.- Background :...
that allowed the large scale manufacture of fluorocarbons required for the Manhattan project.
The Fowler Process
The Fowler processFowler process
The Fowler Process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt fluoride.- Background :...
uses cobalt fluoride to moderate the reaction. In the laboratory, this is typically done in two stages, the first stage being fluorination of cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
difluoride to cobalt trifluoride.
- 2 CoF2 + F2 → 2 CoF3
During the second stage, in this instance to make perfluorohexane
Perfluorohexane
Perfluorohexane or tetradecafluorohexane, is a fluorocarbon. It is a derivative of hexane in which all of the hydrogen atoms are replaced by fluorine atoms. It is used in one formulation of the electronic cooling liquid/insulator Fluorinert for low temperature applications due to its low boiling...
, the hydrocarbon feed is introduced and is fluorinated by the cobalt trifluoride, which is converted back to cobalt difluoride. Both stages are performed at high temperature.
- C6H14 + 28 CoF3 → C6F14 + 14 HF + 28 CoF2
Industrially, both steps are combined, for example in the manufacture of the Flutec range of fluorocarbons, using a vertical stirred bed reactor, with hydrocarbon introduced at the bottom, and fluorine introduced half way up the reactor. The fluorocarbon vapor is recovered from the top.
Electrochemical fluorination
An alternative technique, electrochemical fluorinationElectrochemical fluorination
Electrochemical fluorination , or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds. The general approach represents an application of electrosynthesis...
(ECF) (also known as the Simons' process) involves electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of a substrate dissolved in hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
. As fluorine is itself manufactured by the electrolysis of hydrogen fluoride, this is a rather more direct route to fluorocarbons. The process is run at low voltage (5 - 6 V) so that free fluorine is not liberated. The choice of substrate is restricted as ideally it should be soluble in hydrogen fluoride. Ethers and tertiary amines are typically employed. To make perfluorohexane, trihexylamine is used, for example:
- 2 N(C6H13)3 + 90 HF → 6 C6F14 + 2 NF3 + 45 H2
The perfluorinated amine will also be produced:
- N(C6H13)3 + 42 HF → 2 N(C6F13)3 + 21H2
Both of these products, and others, are manufactured by 3M
3M
3M Company , formerly known as the Minnesota Mining and Manufacturing Company, is an American multinational conglomerate corporation based in Maplewood, Minnesota, United States....
as part of the Fluorinert
Fluorinert
Fluorinert is the trademarked brand name for the line of electronics coolant liquids sold commercially by 3M. It is an electrically insulating, stable fluorocarbon-based fluid which is used in various cooling applications. It is mainly used for cooling electronics...
range.
Derivatives
Fluorocarbon derivatives are highly fluorinated molecules that can be commonly referred to as fluorocarbons. They are economically useful because they share part or nearly all of the properties of fluorocarbons. Some fluorocarbon derivatives have markedly different properties than fluorocarbons. For example, fluorosurfactantFluorosurfactant
Fluorosurfactants, or fluorinated surfactants, are synthetic organofluorine chemical compounds that have multiple fluorine atoms. They can be polyfluorinated or fluorocarbon-based . As surfactants, they are more effective at lowering the surface tension of water than comparable hydrocarbon...
s powerfully reduce surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
by concentrating at the liquid-air interface due to the lipophobicity of fluorocarbons, due to the polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
added to the fluorocarbon chain. Other groups or atoms for fluorocarbon based compounds the oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atom incorporated into an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
group for anesthetics, and the chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
atom for chlorofluorocarbons (CFCs). In a sharp contrast to true fluorocarbons, the chlorine atom produces a chlorine radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
which degrades ozone
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
.
Fluorosurfactants
- Perfluorooctanesulfonic acid (PFOS)
- Perfluorooctanoic acidPerfluorooctanoic acidPerfluorooctanoic acid , also known as C8 and perfluorooctanoate, is a synthetic, stable perfluorinated carboxylic acid and fluorosurfactant. One industrial application is as a surfactant in the emulsion polymerization of fluoropolymers. It has been used in the manufacture of such prominent...
(PFOA) - Perfluorononanoic acidPerfluorononanoic acidPerfluorononanoic acid, or PFNA, is a synthetic perfluorinated carboxylic acid and fluorosurfactant that is also an environmental contaminant found in people and wildlife along with PFOS and PFOA.-Chemistry and properties:...
Anesthetics
- MethoxyfluraneMethoxyfluraneMethoxyflurane is a halogenated ether that was in clinical use as an volatile inhalational anesthetic from its introduction by Joseph F. Artusio et al in 1960 until around 1974. It was first synthesized in the late 1940s by William T...
(contains chlorine) - EnfluraneEnfluraneEnflurane is a halogenated ether that was commonly used for inhalational anesthesia during the 1970s and 1980s. Developed by Ross Terrell in 1963, it was first used clinically in 1966....
(contains chlorine) - IsofluraneIsofluraneIsoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
(contains chlorine) - SevofluraneSevofluraneSevoflurane , also called fluoromethyl hexafluoroisopropyl ether, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used for induction and maintenance of general anesthesia. Together with desflurane, it is replacing isoflurane and halothane in modern anesthesiology...
- DesfluraneDesfluraneDesflurane is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane and isoflurane, it is a racemic mixture of and optical isomers...
Halogenated derivatives
- PolychlorotrifluoroethylenePolychlorotrifluoroethylenePolychlorotrifluoroethylene is a fluoropolymer with the molecular formula n. It is chemically related to PTFE....
([CFClCF2]n) - Perfluorooctyl bromide (Perflubron)
- DichlorodifluoromethaneDichlorodifluoromethaneDichlorodifluoromethane , is a colorless gas, and usually sold under the brand name Freon-12, is a chlorofluorocarbon halomethane , used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in the United States along with many other...
- ChlorodifluoromethaneChlorodifluoromethaneChlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon . This colorless gas is better known as HCFC-22, or R-22. It was once commonly used as a propellant and in air conditioning applications...
Hydrofluorocarbons
- Polyvinylidene fluoridePolyvinylidene fluoridePolyvinylidene fluoride, or PVDF is a highly non-reactive and pure thermoplastic fluoropolymer.PVDF is a specialty plastic material in the fluoropolymer family; it is used generally in applications requiring the highest purity, strength, and resistance to solvents, acids, bases and heat and low...
([CH2CF2]n) - Tetrafluoroethane
Environmental and health concerns
Despite the presence of some natural fluorocarbons such as tetrafluoromethane, which has been reported in rocks, man-made fluorocarbons are potent greenhouse gases.Another important aspect in terms of environmental concers, is certain fluorocarbons' bioaccumulative properties. Fluorocarbons are extremely stable and can be stored in the bodies of both humans and animals. Examples of harmful fluorocarbons include PFOA (perfluorooctanoic acid) and PFOS (perfluorooctane sulfonate), frequently present in water resistant textiles and sprays conferring water resistant properties to textiles. Data from animal studies of PFOA indicate that it can cause several types of tumors and neonatal death and may have toxic effects on the immune, liver, and endocrine systems. Data on the human health effects of PFOA are however sparse.
The fluorocarbon, PFOA and PFOS have both been subject for numerous investigations by the EU and the United States Environmental Protection Agency
United States Environmental Protection Agency
The U.S. Environmental Protection Agency is an agency of the federal government of the United States charged with protecting human health and the environment, by writing and enforcing regulations based on laws passed by Congress...
(EPA) regarding them being harmful to the environment.
External links
- Fluorocarbons and Sulphur Hexafluoride, proposed by the European Fluorocarbons Technical Committee
- CFCs and Ozone Depletion Freeview video provided by the Vega Science Trust.
- Introduction to fluoropolymers
- Organofluorine chemistry by Graham Sandford

