
Continuous distillation
Encyclopedia


Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
, is an ongoing separation in which a mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams. A distillation is the separation
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
or partial separation of a liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
feed mixture into components or fractions by selective boiling
Boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environmental pressure. While below the boiling point a liquid...
(or evaporation
Evaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
) and condensation
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
. A distillation produces at least two output fractions. These fractions include at least one volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms (or residuum) fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
An alternative to continuous distillation is batch distillation
Batch distillation
Batch distillation refers to the use of distillation in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated...
, where the mixture is added to the unit at the start of the distillation, distillate fractions are taken out sequentially in time (one after another) during the distillation, and the remaining bottoms fraction is removed at the end. Because each of the distillate fractions are taken out at different times, only one distillate exit point (location) is needed for a batch distillation and the distillate can just be switched to a different receiver, a fraction-collecting container. Batch distillation is often used when smaller quantities are distilled. In a continuous distillation, each of the fraction streams is taken simultaneously throughout operation; therefore, a separate exit point is needed for each fraction. In practice when there are multiple distillate fractions, each of the distillate exit points are located at different heights on a fractionating column
Fractionating column
A fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities...
. The bottoms fraction can be taken from the bottom of the distillation column or unit, but is often taken from a reboiler
Reboiler
Reboilers are heat exchangers typically used to provide heat to the bottom of industrial distillation columns. They boil the liquid from the bottom of a distillation column to generate vapors which are returned to the column to drive the distillation separation....
connected to the bottom of the column.
Each fraction may contain one or more components (types of chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s). When distilling crude oil or a similar feedstock, each fraction contains many components of similar volatility and other properties. Although it is possible to run a small-scale or laboratory continuous distillation, most often continuous distillation is used in a large-scale industrial process.
Industrial application
Distillation is one of the unit operations of chemical engineeringChemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
. Continuous distillation is used widely in the chemical process industries where large quantities of liquids have to be distilled. Such industries are the natural gas processing
Natural gas processing
Natural-gas processing is a complex industrial process designed to clean raw natural gas by separating impurities and various non-methane hydrocarbons and fluids to produce what is known as pipeline quality dry natural gas.-Background:...
, petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
production, coal tar
Coal tar
Coal tar is a brown or black liquid of extremely high viscosity, which smells of naphthalene and aromatic hydrocarbons. Coal tar is among the by-products when coal iscarbonized to make coke or gasified to make coal gas...
processing, liquor production
Distilled beverage
A distilled beverage, liquor, or spirit is an alcoholic beverage containing ethanol that is produced by distilling ethanol produced by means of fermenting grain, fruit, or vegetables...
, liquified air
Liquid air
Liquid air is air that has been cooled to very low temperatures so that it has condensed to a pale blue mobile liquid. To protect it from room temperature, it must be kept in a vacuum flask. Liquid air can absorb heat rapidly and revert to its gaseous state...
separation, hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
solvents production and similar industries, but it finds its widest application in petroleum refineries
Oil refinery
An oil refinery or petroleum refinery is an industrial process plant where crude oil is processed and refined into more useful petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas...
. In such refineries, the crude oil feedstock is a very complex multicomponent mixture that must be separated and yields of pure chemical compounds are not expected, only groups of compounds within a relatively small range of boiling points
Boiling Points
Boiling Points is a prank reality television show, much like the format used on Candid Camera. It is broadcast on MTV in the United States. In each half-hour episode, annoying situations are set up and deliberately inflicted on one or more young adults who are unaware that they are being tested...
, which are called fractions. These fractions are the origin of the term fractional distillation or fractionation. It is often not worthwhile separating the components in these fractions any further based on product requirements and economics.
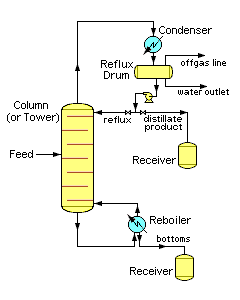
Industrial distillation is typically performed in large, vertical cylindrical columns (as shown in images 1 and 2) known as "distillation towers" or "distillation columns" with diameters ranging from about 65 centimeters to 11 meters and heights ranging from about 6 meters to 60 meters or more.
Principle
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
into a liquid, it becomes richer in the lower boiling component(s) of the original mixture.
This is what happens in a continuous distillation column. A mixture is heated up, and routed into the distillation column. On entering the column, the feed starts flowing down but part of it, the component(s) with lower boiling point(s), vaporizes and rises. However, as it rises, it cools and while part of it continues up as vapor, some of it (enriched in the less volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
component) begins to descend again.
Image 3 depicts a simple continuous fractional distillation tower for separating a feed stream into two fractions, an overhead distillate product and a bottoms product. The "lightest" products (those with the lowest boiling point or highest volatility) exit from the top of the columns and the "heaviest" products (the bottoms, those with the highest boiling point) exit from the bottom of the column. The overhead stream may be cooled and condensed using a water-cooled or air-cooled condenser
Condenser (heat transfer)
In systems involving heat transfer, a condenser is a device or unit used to condense a substance from its gaseous to its liquid state, typically by cooling it. In so doing, the latent heat is given up by the substance, and will transfer to the condenser coolant...
. The bottoms reboiler
Reboiler
Reboilers are heat exchangers typically used to provide heat to the bottom of industrial distillation columns. They boil the liquid from the bottom of a distillation column to generate vapors which are returned to the column to drive the distillation separation....
may be a steam-heated or hot oil-heated heat exchanger
Heat exchanger
A heat exchanger is a piece of equipment built for efficient heat transfer from one medium to another. The media may be separated by a solid wall, so that they never mix, or they may be in direct contact...
, or even a gas or oil-fired furnace
Furnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
.
In a continuous distillation, the system is kept in a steady state
Steady state
A system in a steady state has numerous properties that are unchanging in time. This implies that for any property p of the system, the partial derivative with respect to time is zero:...
or approximate steady state. Steady state means that quantities related to the process do not change as time passes during operation. Such constant quantities include feed input rate, output stream rates, heating and cooling rates, reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
ratio, and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
s, pressures, and compositions at every point (location). Unless the process is disturbed due to changes in feed, heating, ambient temperature, or condensing, steady state is normally maintained. This is also the main attraction of continuous distillation, apart from the minimum amount of (easily instrumentable) surveillance; if the feed rate and feed composition are kept constant, product rate and quality are also constant. Even when a variation in conditions occurs, modern process control
Process control
Process control is a statistics and engineering discipline that deals with architectures, mechanisms and algorithms for maintaining the output of a specific process within a desired range...
methods are commonly able to gradually return the continuous process to another steady state again.
Since a continuous distillation unit is fed constantly with a feed mixture and not filled all at once like a batch distillation, a continuous distillation unit does not need a sizable distillation pot, vessel, or reservoir for a batch fill. Instead, the mixture can be fed directly into the column, where the actual separation occurs. The height of the feed point along the column can vary on the situation and is designed so as to provide optimal results. See McCabe-Thiele method
McCabe-Thiele method
The McCabe-Thiele method was presented by two graduate students at Massachusetts Institute of Technology , Warren L. McCabe and Ernest W. Thiele in 1925. The technique is considered to be the simplest and perhaps most instructive method for analysis of binary distillation...
.
A continuous distillation is often a fractional distillation
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
and can be a vacuum distillation
Vacuum distillation
Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor pressure causing evaporation of the most volatile liquid...
or a steam distillation
Steam distillation
Steam distillation is a special type of distillation for temperature sensitive materials like natural aromatic compounds....
.
Design and operation
Design and operation of a distillation column depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the McCabe-Thiele methodMcCabe-Thiele method
The McCabe-Thiele method was presented by two graduate students at Massachusetts Institute of Technology , Warren L. McCabe and Ernest W. Thiele in 1925. The technique is considered to be the simplest and perhaps most instructive method for analysis of binary distillation...
or the Fenske equation
Fenske equation
The Fenske equation in continuous fractional distillation is an equation used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a fractionation column that is being operated at total reflux .The equation was derived by Merrell Fenske in...
can be used to assist in the design. For a multi-component feed, computerized simulation
Simulation
Simulation is the imitation of some real thing available, state of affairs, or process. The act of simulating something generally entails representing certain key characteristics or behaviours of a selected physical or abstract system....
models are used both for design and subsequently in operation of the column as well. Modeling is also used to optimize already erected columns for the distillation of mixtures other than those the distillation equipment was originally designed for.
When a continuous distillation column is in operation, it has to be closely monitored for changes in feed composition, operating temperature
Operating temperature
An operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
and product composition. Many of these tasks are performed using advanced computer control equipment.
Column feed
The column can be fed in different ways. If the feed is from a source at a pressure higher than the distillation column pressure, it is simply piped into the column. Otherwise, the feed is pumped or compressed into the column. The feed may be a superheated vaporSuperheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
, a saturated vapor, a partially vaporized liquid-vapor mixture, a saturated liquid (i.e., liquid at its boiling point at the column's pressure), or a sub-cooled liquid. If the feed is a liquid at a much higher pressure than the column pressure and flows through a pressure let-down valve just ahead of the column, it will immediately expand and undergo a partial flash vaporization
Flash evaporation
Flash evaporation is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations...
resulting in a liquid-vapor mixture as it enters the distillation column.
Improving separation
Reboiler
Reboilers are heat exchangers typically used to provide heat to the bottom of industrial distillation columns. They boil the liquid from the bottom of a distillation column to generate vapors which are returned to the column to drive the distillation separation....
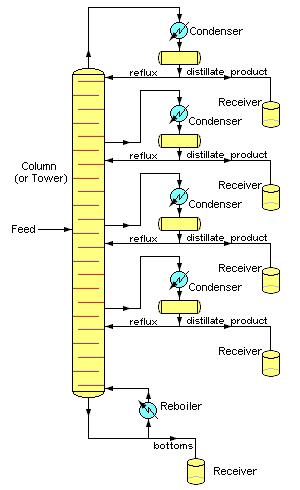
, and the purity of the top product can be improved by recycling some of the externally condensed top product liquid as reflux. Depending on their purpose, distillation columns may have liquid outlets at intervals up the length of the column as shown in image 4.
Reflux
Large-scale industrial fractionation towers use refluxReflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
to achieve more efficient separation of products. Reflux refers to the portion of the condensed overhead liquid product from a distillation tower that is returned to the upper part of the tower as shown in images 3 and 4. Inside the tower, the downflowing reflux liquid provides cooling and partial condensation of the upflowing vapors, thereby increasing the efficacy of the distillation tower. The more reflux that is provided, the better is the tower's separation of the lower boiling from the higher boiling components of the feed. A balance of heating with a reboiler at the bottom of a column and cooling by condensed reflux at the top of the column maintains a temperature gradient (or gradual temperature difference) along the height of the column to provide good conditions for fractionating the feed mixture. Reflux flows at the middle of the tower are called pumparounds.
Changing the reflux (in combination with changes in feed and product withdrawal) can also be used to improve the separation properties of a continuous distillation column while in operation (in contrast to adding plates or trays, or changing the packing, which would, at a minimum, require quite significant downtime).
Plates or trays
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
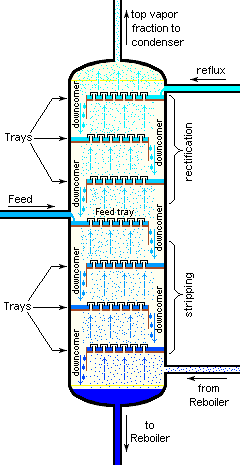
for each feed component in the tower reacts in its unique way to the different pressure and temperature conditions at each of the stages. That means that each component establishes a different concentration in the vapor and liquid phases at each of the stages, and this results in the separation of the components. Some example trays are depicted in image 5. A more detailed, expanded image of two trays can be seen in the theoretical plate
Theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
article. The reboiler often acts as an additional equilibrium stage.
If each physical tray or plate were 100% efficient, then the number of physical trays needed for a given separation would equal the number of equilibrium stages or theoretical plates. However, that is very seldom the case. Hence, a distillation column needs more plates than the required number of theoretical vapor-liquid equilibrium stages.
Packing
Another way of improving the separation in a distillation column is to use a packing materialPacked bed
In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packing can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing...
instead of trays. These offer the advantage of a lower pressure drop across the column (when compared to plates or trays), beneficial when operating under vacuum. If a distillation tower uses packing instead of trays, the number of necessary theoretical equilibrium stages is first determined and then the packing height equivalent to a theoretical equilibrium stage
Theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
, known as the height equivalent to a theoretical plate (HETP), is also determined. The total packing height required is the number theoretical stages multiplied by the HETP.
This packing material can either be random dumped packing such as Raschig ring
Raschig ring
Raschig rings are pieces of tube used in large numbers as a packed bed within columns for distillations and other chemical engineering processes. They are usually ceramic or metal and provide a large surface area within the volume of the column for interaction between liquid and gas or vapour...
s or structured sheet metal
Structured packing
The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns and chemical reactors...
. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
takes place. Unlike conventional tray distillation in which every tray represents a separate point of vapor-liquid equilibrium, the vapor-liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns it is useful to compute a number of theoretical plates to denote the separation efficiency of the packed column with respect to more traditional trays. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
Another factor in addition to the packing shape and surface area that affects the performance of random or structured packing is liquid and vapor distribution entering the packed bed. The number of theoretical stages
Theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
required to make a given separation is calculated using a specific vapor to liquid ratio. If the liquid and vapor are not evenly distributed across the superficial tower area as it enters the packed bed, the liquid to vapor ratio will not be correct in the packed bed and the required separation will not be achieved. The packing will appear to not be working properly. The height equivalent to a theoretical plate (HETP) will be greater than expected. The problem is not the packing itself but the mal-distribution of the fluids entering the packed bed. Liquid mal-distribution is more frequently the problem than vapor. The design of the liquid distributors used to introduce the feed and reflux to a packed bed is critical to making the packing perform at maximum efficiency. Methods of evaluating the effectiveness of a liquid distributor can be found in references.
Overhead system arrangements
Images 4 and 5 assume an overhead stream that is totally condensed into a liquidLiquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
product using water or air-cooling. However, in many cases, the tower overhead is not easily condensed totally and the reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
drum must include a vent gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
outlet stream. In yet other cases, the overhead stream may also contain water vapor because either the feed stream contains some water or some steam is injected into the distillation tower (which is the case in the crude oil distillation towers in oil refineries
Oil refinery
An oil refinery or petroleum refinery is an industrial process plant where crude oil is processed and refined into more useful petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas...
). In those cases, if the distillate product is insoluble in water, the reflux drum may contain a condensed liquid distillate phase, a condensed water phase and a non-condensible gas phase, which makes it necessary that the reflux drum also have a water outlet stream.
Continuous distillation of crude oil
PetroleumPetroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
crude oils contain hundreds of different hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
compounds: paraffin
Paraffin
In chemistry, paraffin is a term that can be used synonymously with "alkane", indicating hydrocarbons with the general formula CnH2n+2. Paraffin wax refers to a mixture of alkanes that falls within the 20 ≤ n ≤ 40 range; they are found in the solid state at room temperature and begin to enter the...
s, naphthenes and aromatics as well as organic sulfur compounds, organic nitrogen compounds and some oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
containing hydrocarbons such as phenols
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group...
. Although crude oils generally do not contain olefins, they are formed in many of the processes used in a petroleum refinery.
The crude oil fractionator does not produce products having a single boiling point; rather, it produces fractions having boiling ranges. For example, the crude oil fractionator produces an overhead fraction called "naphtha
Naphtha
Naphtha normally refers to a number of different flammable liquid mixtures of hydrocarbons, i.e., a component of natural gas condensate or a distillation product from petroleum, coal tar or peat boiling in a certain range and containing certain hydrocarbons. It is a broad term covering among the...
" which becomes a gasoline component after it is further processed through a catalytic hydrodesulfurizer to remove sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and a catalytic reformer to reform
Reform
Reform means to put or change into an improved form or condition; to amend or improve by change of color or removal of faults or abuses, beneficial change, more specifically, reversion to a pure original state, to repair, restore or to correct....
its hydrocarbon molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s into more complex molecules with a higher octane rating
Octane rating
Octane rating or octane number is a standard measure of the anti-knock properties of a motor or aviation fuel. The higher the octane number, the more compression the fuel can withstand before detonating...
value.
The naphtha cut, as that fraction is called, contains many different hydrocarbon compounds. Therefore it has an initial boiling point of about 35 °C and a final boiling point of about 200 °C. Each cut produced in the fractionating columns has a different boiling range. At some distance below the overhead, the next cut is withdrawn from the side of the column and it is usually the jet fuel cut, also known as a kerosene
Kerosene
Kerosene, sometimes spelled kerosine in scientific and industrial usage, also known as paraffin or paraffin oil in the United Kingdom, Hong Kong, Ireland and South Africa, is a combustible hydrocarbon liquid. The name is derived from Greek keros...
cut. The boiling range of that cut is from an initial boiling point of about 150 °C to a final boiling point of about 270 °C, and it also contains many different hydrocarbons. The next cut further down the tower is the diesel oil cut with a boiling range from about 180 °C to about 315 °C. The boiling ranges between any cut and the next cut overlap because the distillation separations are not perfectly sharp. After these come the heavy fuel oil cuts and finally the bottoms product, with very wide boiling ranges. All these cuts are processed further in subsequent refining processes.
See also
- Azeotropic distillationAzeotropic distillationIn chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, azeotropic distillation usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous...
- Extractive distillationExtractive distillationExtractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity...
- Fractional distillationFractional distillationFractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
- Fractionating columnFractionating columnA fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities...
- Steam distillationSteam distillationSteam distillation is a special type of distillation for temperature sensitive materials like natural aromatic compounds....
External links
- Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad, Norwegian University of Science and Technology, Norway
- Distillation, An Introduction by Ming Tham, Newcastle University, UK
- Distillation by the Distillation Group, USA
- Distillation Lecture Notes by Prof. Randall M. Price at Christian Brothers University
- Petroleum Distillation by Wayne Pafco
- Distillation simulation software

