Theoretical plate
Encyclopedia
A theoretical plate in many separation process
es is a hypothetical zone or stage in which two phases, such as the liquid
and vapor
phases of a substance, establish an equilibrium
with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical tray. The performance of many separation processes depends on having a series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficacy of the separation process be it either a distillation
, absorption, chromatographic
, adsorption
or similar process.
processes has been discussed in many reference texts. Any physical device that provides good contact between the vapor and liquid phases present in industrial-scale distillation columns
or laboratory-scale glassware distillation columns constitutes a "plate" or "tray". Since an actual, physical plate is rarely a 100% efficient equilibrium stage, the number of actual plates is more than the required theoretical plates.
So-called bubble-cap or valve-cap trays are examples of the vapor and liquid contact devices used in industrial distillation columns. Another example of vapor and liquid contact devices are the spikes in laboratory Vigreux fractionating columns.
The trays or plates used in industrial distillation columns are fabricated of circular steel plates and usually installed inside the column at intervals of about 60 to 75 cm (24 to 30 inches) up the height of the column. That spacing is chosen primarily for ease of installation and ease of access for future repair or maintenance.
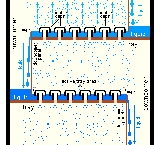
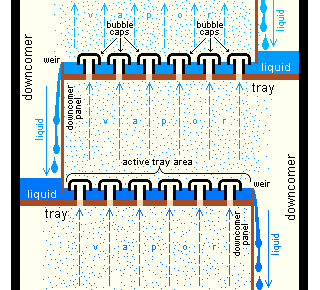 An example of a very simple tray is a perforated tray. The desired contacting between vapor and liquid occurs as the vapor, flowing upwards through the perforations, comes into contact with the liquid flowing downwards through the perforations. In current modern practice, as shown in the adjacent diagram, better contacting is achieved by installing bubble-caps or valve caps at each perforation to promote the formation of vapor bubbles flowing through a thin layer of liquid maintained by a weir
An example of a very simple tray is a perforated tray. The desired contacting between vapor and liquid occurs as the vapor, flowing upwards through the perforations, comes into contact with the liquid flowing downwards through the perforations. In current modern practice, as shown in the adjacent diagram, better contacting is achieved by installing bubble-caps or valve caps at each perforation to promote the formation of vapor bubbles flowing through a thin layer of liquid maintained by a weir
on each tray.
To design a distillation unit or a similar chemical process, the number of theoretical trays or plates (that is, hypothetical equilibrium stages), N t, required in the process should be determined, taking into account a likely range of feedstock composition and the desired degree of separation of the components in the output fractions. In industrial continuous fractionating columns, N t is determined by starting at either the top or bottom of the column and calculating material balances, heat balances and equilibrium flash vaporization
s for each of the succession of equilibrium stages until the desired end product composition is achieved. The calculation process requires the availability of a great deal of vapor-liquid equilibrium
data for the components present in the distillation feed, and the calculation procedure is very complex.
In an industrial distillation column, the N t required to achieve a given separation also depends upon the amount of reflux
used. Using more reflux decreases the number of plates required and using less reflux increases the number of plates required. Hence, the calculation of N t is usually repeated at various reflux rates. N t is then divided by the tray efficiency, E, to determine the actual number of trays or physical plates, Na, needed in the separating column. The final design choice of the number of trays to be installed in an industrial distillation column is then selected based upon an economic balance between the cost of additional trays and the cost of using a higher reflux rate.
There is a very important distinction between the theoretical plate terminology used in discussing conventional distillation trays and the theoretical plate terminology used in the discussions below of packed bed distillation or absorption or in chromatography or other applications. The theoretical plate in conventional distillation trays has no "height". It is simply a hypothetical equilibrium stage. However, the theoretical plate in packed beds, chromatography and other applications is defined as having a height.
s for vapor and liquid contacting have an equivalent concept referred to as the plate height or the height equivalent to a theoretical plate (HETP). HETP arises from the same concept of equilibrium stages as does the theoretical plate and is numerically equal to the absorption bed length divided by the number of theoretical plates in the absorption bed (and in practice is measured in this way).
The material in packed beds can either be random dumped packing (1-3" wide) such as Raschig ring
s or structured sheet metal
. Liquids tend to wet the surface of the packing and the vapors contact the wetted surface, where mass transfer
occurs.
processes by Martin
and Synge. The IUPAC's Gold Book
provides a definition of the number of theoretical plates in a chromatography column.
The same equation applies in chromatography processes as for the packed bed processes, namely:
In chromatography, the HETP may also be calculated with the Van Deemter equation.
and some types of adsorption
.
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
es is a hypothetical zone or stage in which two phases, such as the liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
phases of a substance, establish an equilibrium
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical tray. The performance of many separation processes depends on having a series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficacy of the separation process be it either a distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
, absorption, chromatographic
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
, adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
or similar process.
Applications
The concept of theoretical plates and trays or equilibrium stages is used in the design of many different types of separation.Distillation columns
The concept of theoretical plates in designing distillationDistillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
processes has been discussed in many reference texts. Any physical device that provides good contact between the vapor and liquid phases present in industrial-scale distillation columns
Fractionating column
A fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities...
or laboratory-scale glassware distillation columns constitutes a "plate" or "tray". Since an actual, physical plate is rarely a 100% efficient equilibrium stage, the number of actual plates is more than the required theoretical plates.
| where: | |
 |
= the number of actual, physical plates or trays |
|---|---|
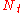 |
= the number of theoretical plates or trays |
 |
= the plate or tray efficiency |
So-called bubble-cap or valve-cap trays are examples of the vapor and liquid contact devices used in industrial distillation columns. Another example of vapor and liquid contact devices are the spikes in laboratory Vigreux fractionating columns.
The trays or plates used in industrial distillation columns are fabricated of circular steel plates and usually installed inside the column at intervals of about 60 to 75 cm (24 to 30 inches) up the height of the column. That spacing is chosen primarily for ease of installation and ease of access for future repair or maintenance.

Weir
A weir is a small overflow dam used to alter the flow characteristics of a river or stream. In most cases weirs take the form of a barrier across the river that causes water to pool behind the structure , but allows water to flow over the top...
on each tray.
To design a distillation unit or a similar chemical process, the number of theoretical trays or plates (that is, hypothetical equilibrium stages), N t, required in the process should be determined, taking into account a likely range of feedstock composition and the desired degree of separation of the components in the output fractions. In industrial continuous fractionating columns, N t is determined by starting at either the top or bottom of the column and calculating material balances, heat balances and equilibrium flash vaporization
Flash evaporation
Flash evaporation is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations...
s for each of the succession of equilibrium stages until the desired end product composition is achieved. The calculation process requires the availability of a great deal of vapor-liquid equilibrium
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
data for the components present in the distillation feed, and the calculation procedure is very complex.
In an industrial distillation column, the N t required to achieve a given separation also depends upon the amount of reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
used. Using more reflux decreases the number of plates required and using less reflux increases the number of plates required. Hence, the calculation of N t is usually repeated at various reflux rates. N t is then divided by the tray efficiency, E, to determine the actual number of trays or physical plates, Na, needed in the separating column. The final design choice of the number of trays to be installed in an industrial distillation column is then selected based upon an economic balance between the cost of additional trays and the cost of using a higher reflux rate.
There is a very important distinction between the theoretical plate terminology used in discussing conventional distillation trays and the theoretical plate terminology used in the discussions below of packed bed distillation or absorption or in chromatography or other applications. The theoretical plate in conventional distillation trays has no "height". It is simply a hypothetical equilibrium stage. However, the theoretical plate in packed beds, chromatography and other applications is defined as having a height.
Distillation and absorption packed beds
Distillation and absorption separation processes using packed bedPacked bed
In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packing can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing...
s for vapor and liquid contacting have an equivalent concept referred to as the plate height or the height equivalent to a theoretical plate (HETP). HETP arises from the same concept of equilibrium stages as does the theoretical plate and is numerically equal to the absorption bed length divided by the number of theoretical plates in the absorption bed (and in practice is measured in this way).
| where: | |
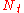 |
= the number of theoretical plates (also called the "plate count") |
|---|---|
 |
= the total bed height |
 |
= the height equivalent to a theoretical plate |
The material in packed beds can either be random dumped packing (1-3" wide) such as Raschig ring
Raschig ring
Raschig rings are pieces of tube used in large numbers as a packed bed within columns for distillations and other chemical engineering processes. They are usually ceramic or metal and provide a large surface area within the volume of the column for interaction between liquid and gas or vapour...
s or structured sheet metal
Structured packing
The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns and chemical reactors...
. Liquids tend to wet the surface of the packing and the vapors contact the wetted surface, where mass transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
occurs.
Chromatographic processes
The theoretical plate concept was also adapted for chromatographicChromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
processes by Martin
Archer John Porter Martin
Archer John Porter Martin, FRS was a British chemist who shared the 1952 Nobel Prize in Chemistry for the invention of partition chromatography with Richard Synge....
and Synge. The IUPAC's Gold Book
Gold Book
The Compendium of Chemical Terminology is a book published by the International Union of Pure and Applied Chemistry containing internationally accepted definitions for terms in chemistry...
provides a definition of the number of theoretical plates in a chromatography column.
The same equation applies in chromatography processes as for the packed bed processes, namely:
| where: | |
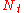 |
= the number of theoretical plates (also called the "plate count") |
|---|---|
 |
= the total column length |
 |
= the height equivalent to a theoretical plate |
In chromatography, the HETP may also be calculated with the Van Deemter equation.
Other applications
The concept of theoretical plates or trays applies to other processes as well, such as capillary electrophoresisCapillary electrophoresis
Capillary electrophoresis , also known as capillary zone electrophoresis , can be used to separate ionic species by their charge and frictional forces and hydrodynamic radius. In traditional electrophoresis, electrically charged analytes move in a conductive liquid medium under the influence of an...
and some types of adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
.
See also
- Batch distillationBatch distillationBatch distillation refers to the use of distillation in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated...
- Continuous distillationContinuous distillationContinuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. A distillation is the separation or partial separation of a liquid feed mixture into components or...
- Extractive distillationExtractive distillationExtractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity...
- Fenske equationFenske equationThe Fenske equation in continuous fractional distillation is an equation used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a fractionation column that is being operated at total reflux .The equation was derived by Merrell Fenske in...
- Fractional distillationFractional distillationFractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
- McCabe-Thiele MethodMcCabe-Thiele methodThe McCabe-Thiele method was presented by two graduate students at Massachusetts Institute of Technology , Warren L. McCabe and Ernest W. Thiele in 1925. The technique is considered to be the simplest and perhaps most instructive method for analysis of binary distillation...
External links
- Distillation, An Introduction by Ming Tham, Newcastle University, UK
- Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad, Norwegian University of Science and Technology, Norway




