
Basic concepts of quantum mechanics
Encyclopedia
- This article is intended as an accessible, non-technical introduction to the subject. For the main encyclopedia article, see Quantum mechanicsQuantum mechanicsQuantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
. For a somewhat more technical introduction to the subject that requires some algebra, see Introduction to quantum mechanicsIntroduction to quantum mechanicsQuantum mechanics is the body of scientific principles that explains the behavior of matter and its interactions with energy on the scale of atoms and atomic particles....
.
Quantum mechanics explains the behaviour of matter
Matter
Matter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume...
and energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
on the scale of atoms and subatomic particles.
Classical physics
Classical physics
What "classical physics" refers to depends on the context. When discussing special relativity, it refers to the Newtonian physics which preceded relativity, i.e. the branches of physics based on principles developed before the rise of relativity and quantum mechanics...
explains matter and energy at the macroscopic level of the scale familiar to human experience, including the behavior of astronomical bodies. It remains the key to measurement
Measure (physics)
The measure in quantum physics is the integration measure used for performing a path integral.In quantum field theory, one must sum over all possible histories of a system....
for much of modern science
Science
Science is a systematic enterprise that builds and organizes knowledge in the form of testable explanations and predictions about the universe...
and technology; but at the end of the 19th Century observers discovered phenomena in both the large (macro) and the small (micro) worlds which classical physics could not explain.
This article describes the limitations of classical physics, and explains the main concepts of the quantum theory which supplanted it in the early decades of the 20th Century. These concepts are described in roughly the order they were first discovered
History of quantum mechanics
The history of quantum mechanics, as it interlaces with the history of quantum chemistry, began essentially with a number of different scientific discoveries: the 1838 discovery of cathode rays by Michael Faraday; the 1859-1860 winter statement of the black body radiation problem by Gustav...
.
Origins in black body radiation

Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation....
is electromagnetic radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
emitted from the surface of an object due to the object's temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. If the object is heated sufficiently, it starts to emit light at the red end of the spectrum
Spectrum
A spectrum is a condition that is not limited to a specific set of values but can vary infinitely within a continuum. The word saw its first scientific use within the field of optics to describe the rainbow of colors in visible light when separated using a prism; it has since been applied by...
: it is red hot. Heating it further causes the color to change, as light of shorter wavelengths (higher frequencies) becomes stronger. A good emitter is also a good absorber. When it is cold, such an object looks black, as it emits practically no visible light, absorbing all light that falls on it. Consequently, its absorbing properties make it an ideal emitter; this is known as a black body
Black body
A black body is an idealized physical body that absorbs all incident electromagnetic radiation. Because of this perfect absorptivity at all wavelengths, a black body is also the best possible emitter of thermal radiation, which it radiates incandescently in a characteristic, continuous spectrum...
, and the radiation it emits is called black body radiation.
In the late 19th Century, thermal radiation had been fairly well characterised experimentally. The wavelength at which the radiation is strongest is given by Wien's displacement law
Wien's displacement law
Wien's displacement law states that the wavelength distribution of thermal radiation from a black body at any temperature has essentially the same shape as the distribution at any other temperature, except that each wavelength is displaced on the graph...
, and the overall power emitted per unit area is given by the Stefan–Boltzmann law. As temperature increases, the glow color changes from red to yellow to white to blue. Even as the peak wavelength moves into the ultra-violet,enough radiation continues to be emitted in the blue wavelengths that the body continues to appear blue. It never becomes invisible—indeed, the radiation of visible light increases monotonically
Monotonic function
In mathematics, a monotonic function is a function that preserves the given order. This concept first arose in calculus, and was later generalized to the more abstract setting of order theory....
with temperature.
Physicists were searching for a theoretical explanation of these experimental results.
The answer from classical physics is called the Rayleigh–Jeans law. This law agrees with experimental results at long wavelengths. At short wavelengths, however, it predicts that energy is emitted by a hot body at an infinite rate. This result, which is clearly wrong, is known as the ultraviolet catastrophe
Ultraviolet catastrophe
The ultraviolet catastrophe, also called the Rayleigh–Jeans catastrophe, was a prediction of late 19th century/early 20th century classical physics that an ideal black body at thermal equilibrium will emit radiation with infinite power....
.

Max Planck
Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came...
in 1900. He modelled the thermal radiation as being in equilibrium by analogy with a set of harmonic oscillators. But to reproduce the experimental results, each oscillator had to have an exact number of energy units, rather than being able to have any arbitrary amount of energy. In other words, the energy of each oscillator was quantised. The word "quantum
Quantum
In physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete...
" comes from the Latin word for "how much" (as does "quantity"). Something that is "quantised", like the energy of Planck's harmonic oscillators, can only have specific values. For example, in most countries money is effectively quantised: the "quantum of money" being the lowest-value coin in circulation. "Mechanics" is the branch of science that deals with the action of forces on objects, and so "quantum mechanics" is the form of mechanics that deals with objects for which particular properties are quantised. He determined that the energy of each oscillator was proportional to the frequency, i.e. that it was always an exact multiple of a constant now known as the Planck constant
Planck constant
The Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
.
Planck's law was the first quantum theory in physics, and Planck won the Nobel Prize in 1918 "in recognition of the services he rendered to the advancement of Physics by his discovery of energy quanta". At the time, however, Planck's own view was that quantisation was purely a mathematical trick to explain the unexpected experimental results, rather than (as we now know) a fundamental change in our understanding of the world.
Photons: the quantisation of light

Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
took an extra step. He suggested that quantisation wasn't just a mathematical trick, but that the energy in a beam of light occurs in individual packets now called photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s. The energy of a single photon is Planck's constant multiplied by the photon's frequency.
Einstein's proposal was able to explain several puzzling properties of the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
, which is the way certain metals give off electrons when light falls on them. For centuries, scientists had debated between two possible theories of light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
: was it a wave or did it instead consist of a stream of tiny particles? By the 19th Century, the debate was generally considered to have been settled in favour of the wave theory, because it was able to explain observed effects such as refraction
Refraction
Refraction is the change in direction of a wave due to a change in its speed. It is essentially a surface phenomenon . The phenomenon is mainly in governance to the law of conservation of energy. The proper explanation would be that due to change of medium, the phase velocity of the wave is changed...
, diffraction
Diffraction
Diffraction refers to various phenomena which occur when a wave encounters an obstacle. Italian scientist Francesco Maria Grimaldi coined the word "diffraction" and was the first to record accurate observations of the phenomenon in 1665...
and polarisation. Because of this, Einstein's proposal was met by great scepticism. Eventually, however, his particle analogy became accepted, as it helped explain how light delivers energy in multiples of certain set values, called quanta of energy. Nevertheless, the wave analogy remained indispensable for helping to explain other phenomena of light, such as diffraction
Diffraction
Diffraction refers to various phenomena which occur when a wave encounters an obstacle. Italian scientist Francesco Maria Grimaldi coined the word "diffraction" and was the first to record accurate observations of the phenomenon in 1665...
.
Thus, for the first time, was an object demonstrating both wave-like and particle-like characteristics modelled as being based upon discrete energy levels.
Bohr model of the atom
By the early 20th century, it was known that atomAtom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s consisted of a diffuse cloud of negatively-charged electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s surrounding a small, dense, positively-charged nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. This suggested a model in which the electrons circled around the nucleus like planets orbiting the sun.The classical model of the atom is called the planetary model or the Rutherford model
Rutherford model
The Rutherford model or planetary model is a model of the atom devised by Ernest Rutherford. Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested on Rutherford's 1911 analysis that the so-called "plum pudding model" of J. J. Thomson of the atom was incorrect...
, after Ernest Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
who proposed it in 1911, based on the Geiger-Marsden gold foil experiment
Geiger-Marsden experiment
The Geiger–Marsden experiment was an experiment to probe the structure of the atom performed by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester...
which first demonstrated the existence of the nucleus. However, it was also known that the atom in this model would be unstable: the orbiting electrons should give off electromagnetic radiation, causing them to lose energy and spiral towards the nucleus, colliding with it in a fraction of a second.
A second, related, puzzle was the emission spectrum
Emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state....
of atoms. When a gas is heated, it gives off light at certain discrete frequencies; for example, the visible light given off by hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
consists of four different colours, as shown in the picture below. In contrast, white light contains light at the whole range of visible frequencies.
In 1913 Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
proposed a new model of the atom that included quantised electron orbits. This solution became known as the Bohr model of the atom. In Bohr's model, electrons could inhabit only particular orbits around the atomic nucleus. When an atom emits or absorbs energy, the electron does not move in a continuous trajectory from one orbit around the nucleus to another, as might be expected in classical theory. Instead, the electron jumps instantaneously from one orbit to another, giving off the difference in energy as light in the form of a photon. The possible energies of the photons given off by each element in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
are determined by the difference in energy between the orbits, so the emission spectrum for each element will contain a number of lines. The Bohr model was able to explain the emission spectrum of hydrogen, but wasn't able to make accurate predictions for multi-electron atoms, or to explain why some spectral lines are brighter than others.
Wave-particle duality
Quantum mechanics is based upon the concept that subatomic particles can have both wave-like and particle-like properties. This phenomenon is known as wave–particle duality. The explanation stems from a theory proposed by French physicist Louis de Broglie in 1924, that subatomic particles such as electrons are associated with waves. Experiments later showed that he was correct: electrons can bend around objects and can display wave shapes.Consequently, neither wave nor particle is an entirely satisfactory model to use in understanding light. Indeed, astrophysicist A.S. Eddington
Arthur Stanley Eddington
Sir Arthur Stanley Eddington, OM, FRS was a British astrophysicist of the early 20th century. He was also a philosopher of science and a popularizer of science...
proposed in 1927 that "We can scarcely describe such an entity as a wave or as a particle; perhaps as a compromise we had better call it a 'wavicle' ". This term was later popularised by mathematician Banesh Hoffmann
Banesh Hoffmann
Banesh Hoffmann was a British mathematician and physicist known for his association with Albert Einstein.-Life:Banesh Hoffmann was born in Richmond, England, on 6 September 1906...
.
The concept of waves and particles, and the analogies which use them, are mechanisms of classical physics
Classical physics
What "classical physics" refers to depends on the context. When discussing special relativity, it refers to the Newtonian physics which preceded relativity, i.e. the branches of physics based on principles developed before the rise of relativity and quantum mechanics...
. Quantum mechanics, which seeks to explain nature at a level underlying that of the atoms which comprise matter, cannot be understood in such terms. The classical concepts presuppose an artificial division of matter (as particles) and energy (as waves) that has no objective validity on the sub-atomic level. If the distinction no longer holds true, it is not surprising if classes of object can exhibit the characteristics of either.
Uncertainty principle

In 1927 German physicist Werner Heisenberg
Werner Heisenberg
Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory...
proved that in the sub-atomic world such assumptions are not correct. Quantum mechanics shows that certain pairs of physical properties, such as position and speed, cannot both be known to arbitrary precision. He showed that the more precisely one of them is known, the less precisely the other can be known. This statement is known as the uncertainty principle
Uncertainty principle
In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
(or Heisenberg's uncertainty principle). It is not a statement about the accuracy of our measuring equipment, but about the nature of the system itself -- our naive assumption that an object has a definite position and speed is incorrect. On a scale of cars and people, these uncertainties are still present but are too small to be noticed; yet they are large enough that when dealing with individual atoms and electrons they become critical.
Heisenberg gave, as an illustration, the measurement of the position and momentum of an electron using a photon of light. In measuring the electron's position, the higher the frequency of the photon the more accurate is the measurement of the position of the impact, but the greater is the disturbance of the electron, which absorbs a random amount of energy, rendering the measurement obtained of its momentum
Momentum
In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
increasingly uncertain (momentum is velocity multiplied by mass), for one is necessarily measuring its post-impact disturbed momentum, from the collision products, not its original momentum. With a photon of lower frequency the disturbance - hence uncertainty - in the momentum is less, but so is the accuracy of the measurement of the position of the impact.
The uncertainty principle shows mathematically that the product of the uncertainty in the position and momentum of a particle can never be less than a certain value, and that this value is related to Planck's constant. It is, in point of fact, up to a small numerical factor equal to Planck's constant.
Schrödinger's wave equation

Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933...
hoped that a theory based on continuous wave-like properties Schrödinger's formulation of quantum mechanics based on waves is sometimes referred to as "wave mechanics", to distinguish it from the matrix mechanics
Matrix mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925.Matrix mechanics was the first conceptually autonomous and logically consistent formulation of quantum mechanics. It extended the Bohr Model by describing how the quantum jumps...
formulation of Heisenberg, Max Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
and Pascual Jordan
Pascual Jordan
-Further reading:...
. could avoid what he called (in the reported words of Wilhelm Wien
Wilhelm Wien
Wilhelm Carl Werner Otto Fritz Franz Wien was a German physicist who, in 1893, used theories about heat and electromagnetism to deduce Wien's displacement law, which calculates the emission of a blackbody at any temperature from the emission at any one reference temperature.He also formulated an...
) "this nonsense about quantum jumps."
Building on De Broglie's theoretical model of particles as waves, Schrödinger accordingly brought forth in 1926 what has been called "the fundamental equation" of quantum mechanics.
In point of fact, Schrödinger's wave equation is as central to quantum mechanics as Einstein's equation
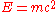 is to Relativity
is to RelativitySpecial relativity
Special relativity is the physical theory of measurement in an inertial frame of reference proposed in 1905 by Albert Einstein in the paper "On the Electrodynamics of Moving Bodies".It generalizes Galileo's...
.
The equation describes the probability waves which govern the motion of sub-atomic particles, "and it specifies how these waves are altered by external influences. Schrödinger established the correctness of the equation by applying it to the hydrogen atom
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
, predicting many of its properties with remarkable accuracy. The equation is used extensively in atomic, nuclear, and solid-state physics
Solid-state physics
Solid-state physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from...
."
Atomic orbital model
Bohr's model of the atom was essentially two-dimensional — an electron orbiting in a plane around its nuclear "sun." In modern theory, orbital has replaced the earlier word orbit concerning the position of an electron in relation to the nucleus of an atomAtomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. It is often depicted as a three-dimensional region within which there is a 95 percent probability of finding the electron.
The uncertainty principle states that an electron cannot be viewed as having an exact location at any given time. The concepts of exact position and exact velocity
Velocity
In physics, velocity is speed in a given direction. Speed describes only how fast an object is moving, whereas velocity gives both the speed and direction of the object's motion. To have a constant velocity, an object must have a constant speed and motion in a constant direction. Constant ...
(distance traveled per unit of time) taken together really have no meaning in nature. An orbital, then, is a "cloud" of possible locations in which an electron might be found, a distribution of probabilities rather than a precise location.
Quantum field theory
Paul Dirac
Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics...
, when he attempted to quantise
Quantization (physics)
In physics, quantization is the process of explaining a classical understanding of physical phenomena in terms of a newer understanding known as "quantum mechanics". It is a procedure for constructing a quantum field theory starting from a classical field theory. This is a generalization of the...
the electromagnetic field
Electromagnetic field
An electromagnetic field is a physical field produced by moving electrically charged objects. It affects the behavior of charged objects in the vicinity of the field. The electromagnetic field extends indefinitely throughout space and describes the electromagnetic interaction...
— a procedure for constructing a quantum theory starting from a classical theory.
A field in physics is "a region or space in which a given effect (such as magnetism
Magnetism
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well...
) exists." Other effects that manifest themselves as fields are gravitation
Gravitation
Gravitation, or gravity, is a natural phenomenon by which physical bodies attract with a force proportional to their mass. Gravitation is most familiar as the agent that gives weight to objects with mass and causes them to fall to the ground when dropped...
and static electricity
Static electricity
Static electricity refers to the build-up of electric charge on the surface of objects. The static charges remain on an object until they either bleed off to ground or are quickly neutralized by a discharge. Static electricity can be contrasted with current electricity, which can be delivered...
. In 2008, physicist Richard Hammond
Richard Hammond (physicist)
Richard Hammond is an Adjunct Professor at the University of North Carolina at Chapel Hill and the author of the book "The Unknown Universe: The Origin of the Universe, Quantum Gravity, Wormholes, and Other Things Science Still Can't Explain". He also works for the United States Army Research...
wrote that
Sometimes we distinguish between quantum mechanics (QM) and quantum field theory (QFT). QM refers to a system in which the number of particles is fixed, and the fields (such as the electromechanical field) are continuous classical entities. QFT . . . goes a step further and allows for the creation and annihilation of particles . . . .
He added, however, that quantum mechanics is often used to refer to "the entire notion of quantum view."
In 1931, Dirac proposed the existence of particles that later became known as anti-matter. Dirac shared the Nobel Prize in physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
for 1933 with Schrödinger
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933...
, "for the discovery of new productive forms of atomic theory
Atomic theory
In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity...
."
Practical use
The main value of the quantum mechanics theory is its practical applications. Examples include the laserLaser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
, the transistor
Transistor
A transistor is a semiconductor device used to amplify and switch electronic signals and power. It is composed of a semiconductor material with at least three terminals for connection to an external circuit. A voltage or current applied to one pair of the transistor's terminals changes the current...
, the electron microscope
Electron microscope
An electron microscope is a type of microscope that uses a beam of electrons to illuminate the specimen and produce a magnified image. Electron microscopes have a greater resolving power than a light-powered optical microscope, because electrons have wavelengths about 100,000 times shorter than...
, and magnetic resonance imaging
Magnetic resonance imaging
Magnetic resonance imaging , nuclear magnetic resonance imaging , or magnetic resonance tomography is a medical imaging technique used in radiology to visualize detailed internal structures...
. The study of semiconductors led to the invention of the diode
Diode
In electronics, a diode is a type of two-terminal electronic component with a nonlinear current–voltage characteristic. A semiconductor diode, the most common type today, is a crystalline piece of semiconductor material connected to two electrical terminals...
and the transistor
Transistor
A transistor is a semiconductor device used to amplify and switch electronic signals and power. It is composed of a semiconductor material with at least three terminals for connection to an external circuit. A voltage or current applied to one pair of the transistor's terminals changes the current...
, which are indispensable for modern electronics
Electronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
.
In even the simple light switch
Light switch
A light switch is a switch, most commonly used to operate electric lights, permanently connected equipment, or electrical outlets. In torches the switch is often near the bulb, but may be in the tail, or even the entire head itself may constitute the switch .-Wall-mounted switches:Switches for...
, quantum tunnelling is vital, as otherwise the electrons in the electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
could not penetrate the potential barrier made up of a layer of oxide. Flash memory
Flash memory
Flash memory is a non-volatile computer storage chip that can be electrically erased and reprogrammed. It was developed from EEPROM and must be erased in fairly large blocks before these can be rewritten with new data...
chips found in USB drives also use quantum tunnelling, to erase their memory cells.
Further reading
- Richard FeynmanRichard FeynmanRichard Phillips Feynman was an American physicist known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics and the physics of the superfluidity of supercooled liquid helium, as well as in particle physics...
, 1985. QED: The Strange Theory of Light and Matter, Princeton University Press. ISBN 0-691-08388-6

