Anionic addition polymerization
Encyclopedia
Anionic addition polymerization is a form of chain-growth polymerization or addition polymerization
that involves the polymerization
of vinyl monomers with strong electronegative groups. This polymerization is carried out through a carbanion
active species. Like all addition polymerizations, it takes place in three steps: chain initiation, chain propagation
, and chain termination
. Living polymerization
s, which lack a formal termination pathway, occur in many anionic addition polymerizations. The advantage of living anionic addition polymerizations is that they allow for the control of structure and composition.
Anionic polymerizations are used in the production of polydiene synthetic rubber
s, solution styrene/butadiene rubbers (SBR), and styrenic thermoplastic elastomers.
and co – workers in 1956 was one of the breakthrough events in the field of polymer science
. When Szwarc learned that the electron transfer
between radical anion of naphthalene
and styrene in an aprotic solvent such as tetrahydrofuran
gave a messy product, he started investigating the reaction in more detail. He proved that the electron transfer results in the formation of a dianion which rapidly added styrene to form a "two – ended living polymer." Being a physical chemist, Szwarc set forth in understanding the mechanism of such living polymerization in greater detail. His work elucidated the kinetics
and the thermodynamics
of the process in considerable detail. At the same time, he explored the structure property relationship of the various ion pairs and radical ions involved. This had great ramifications in future research in polymer synthesis, because Szwarc had found a way to make polymers with greater control over molecular weight, molecular weight distribution and the architecture of the polymer.
The use of alkali metals to initiate polymerization of 1,3-diene
s led to the discovery by Stavely and co-workers at Firestone Tire and Rubber company of cis-1,4-polyisoprene. This sparked the development of commercial anionic polymerization processes that utilize alkyllithium initiatiors.
monomer
s, the substituent
s on the double bond
must be able to stabilize a negative charge. Stabilization occurs through delocalization of the negative charge. Because of the nature of the carbanion
propagating center, substituents that react with bases or nucleophiles either must not be present or be protected.
 Vinyl monomers with substituents that stabilize the negative charge through charge delocalization, undergo polymerization without termination or chain transfer. These monomers include styrene, diene
Vinyl monomers with substituents that stabilize the negative charge through charge delocalization, undergo polymerization without termination or chain transfer. These monomers include styrene, diene
s, methacrylate
, vinyl pyridine
, aldehyde
s, epoxide
, episulfide
, cyclic siloxane
, and lactone
s.
Polar monomers, using controlled conditions and low temperatures, can undergo anionic polymerization. However, at higher temperatures they do not produce living stable, carbanionic chain ends because their polar substituents can undergo side reactions with both initiators and propagating chain centers. The effects of counterion, solvent, temperature, Lewis base additives, and inorganic solvents have been investigated to increase the potential of anionic polymerizations of polar monomers. Polar monomers include acrylonitrile
, cyanoacrylate
, propylene oxide
, vinyl ketone
, acrolein
, vinyl sulfone
, vinyl sulfoxide
, vinyl silane and isocyanate
.
. Polar solvents are necessary for this type of initiation both for stability of the anion-radical and to solvate the cation species formed. The anion-radical can then transfer an electron to the monomer. Initiation can also involve the transfer of an electron from the alkali metal to the monomer to form an anion-radical. Initiation occurs on the surface of the metal, with the reversible transfer of an electron to the adsorbed monomer.
Initiation can also involve the transfer of an electron from the alkali metal to the monomer to form an anion-radical. Initiation occurs on the surface of the metal, with the reversible transfer of an electron to the adsorbed monomer.
s, alkoxide
s, hydroxide
s, cyanide
s, phosphine
s, amine
s and organometallic compounds (alkyllithium compounds and Grignard reagents). The initiation process involves the addition of a neutral (B:) or negative (B:-) nucleophile
to the monomer. The most commercially useful of these initiators has been the alkyllithium initiators. They are primarily used for the polymerization of styrenes and dienes.
The most commercially useful of these initiators has been the alkyllithium initiators. They are primarily used for the polymerization of styrenes and dienes.
 Propagation in anionic addition polymerization results in the complete consumption of monomer. It is very fast and occurs at low temperatures. This is due to the anion not being very stable, the speed of the reaction as well as that heat is released during the reaction. The stability can be greatly enhanced by reducing the temperatures to near 0˚C. The propagation rates are generally fairly high compared to the decay reaction, so the overall polymerization rates is generally not affected.
Propagation in anionic addition polymerization results in the complete consumption of monomer. It is very fast and occurs at low temperatures. This is due to the anion not being very stable, the speed of the reaction as well as that heat is released during the reaction. The stability can be greatly enhanced by reducing the temperatures to near 0˚C. The propagation rates are generally fairly high compared to the decay reaction, so the overall polymerization rates is generally not affected.
, carbon dioxide
or water
. Intentional termination can occur through the addition of water or alcohol. Another method of termination, chain transfer, can occur when an agent can act as a Brønsted acid. In this case, the pKa
value of the agent is similar to the conjugate acid of the propagating carbanionic chain end. Spontaneous termination occurs because the concentration of carbanion centers decay over time and eventually results in hydride elimination.
Polar monomers are more reactive because they are stabilized by their polar substituents. These polar substituents can react with nucleophiles which results in termination as well as side reactions that compete with both initiation and propagation.
One of the remarkable features of living anionic polymerization is that the mechanism involves no formal termination step. In the absence of impurities, the carbanion would still be active and capable of adding another monomer. The chains will remain active indefinitely unless there is inadvertent or deliberate termination or chain transfer.
of anionic addition polymerization depend on whether or not a termination pathway occurs.
Addition polymerization
Chain growth polymerization is a polymerization technique where unsaturated monomer molecules add on to a growing polymer chain one at a time...
that involves the polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
of vinyl monomers with strong electronegative groups. This polymerization is carried out through a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
active species. Like all addition polymerizations, it takes place in three steps: chain initiation, chain propagation
Chain propagation
Chain propagation is a process in which a reactive intermediate is continuously regenerated during the course of a chemical reaction. In polymerization reaction, the reactive end-groups of a polymer chain react in each propagation step with a new monomer molecule transferring the reactive group to...
, and chain termination
Chain termination
Chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.- Mechanisms of Termination :...
. Living polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
s, which lack a formal termination pathway, occur in many anionic addition polymerizations. The advantage of living anionic addition polymerizations is that they allow for the control of structure and composition.
Anionic polymerizations are used in the production of polydiene synthetic rubber
Synthetic rubber
Synthetic rubber is is any type of artificial elastomer, invariably a polymer. An elastomer is a material with the mechanical property that it can undergo much more elastic deformation under stress than most materials and still return to its previous size without permanent deformation...
s, solution styrene/butadiene rubbers (SBR), and styrenic thermoplastic elastomers.
History
As early as 1936, Karl Ziegler proposed that anionic polymerization of styrene and butadiene by consecutive addition of monomer to an alkyl lithium initiator occurred without chain transfer or termination. Twenty years later, living polymerization was demonstrated by Szwarc. The early work of Michael SzwarcMichael Szwarc
Michael Szwarc was a British and American polymer chemist who discovered and studied ionic living polymerization.- Biography :...
and co – workers in 1956 was one of the breakthrough events in the field of polymer science
Polymer science
Polymer science or macromolecular science is the subfield of materials science concerned with polymers, primarily synthetic polymers such as plastics...
. When Szwarc learned that the electron transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
between radical anion of naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
and styrene in an aprotic solvent such as tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
gave a messy product, he started investigating the reaction in more detail. He proved that the electron transfer results in the formation of a dianion which rapidly added styrene to form a "two – ended living polymer." Being a physical chemist, Szwarc set forth in understanding the mechanism of such living polymerization in greater detail. His work elucidated the kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
and the thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
of the process in considerable detail. At the same time, he explored the structure property relationship of the various ion pairs and radical ions involved. This had great ramifications in future research in polymer synthesis, because Szwarc had found a way to make polymers with greater control over molecular weight, molecular weight distribution and the architecture of the polymer.
The use of alkali metals to initiate polymerization of 1,3-diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
s led to the discovery by Stavely and co-workers at Firestone Tire and Rubber company of cis-1,4-polyisoprene. This sparked the development of commercial anionic polymerization processes that utilize alkyllithium initiatiors.
Monomer Characteristics
In order for polymerization to occur with vinylVinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s, the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s on the double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
must be able to stabilize a negative charge. Stabilization occurs through delocalization of the negative charge. Because of the nature of the carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
propagating center, substituents that react with bases or nucleophiles either must not be present or be protected.

Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
s, methacrylate
Methacrylate
Methacrylates are the salts or esters of methacrylic acid.Methacrylates contain methyl-vinyl groups, that is, two carbon atoms double bonded to each other, directly attached to the carbonyl carbon, and wherein the vinyl group is substituted with a non-terminal methyl group.Methacrylates are common...
, vinyl pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
, episulfide
Episulfide
Episulfides are a class of organic compounds that contain a saturated heterocyclic ring consisting of two carbon atoms and one sulfur atom. It is the sulfur analogue of an epoxide or aziridine. They are also known as thiiranes, olefin sulfides, thioalkylene oxides, and thiacyclopropanes.The parent...
, cyclic siloxane
Siloxane
A siloxane is any chemical compound composed of units of the form R2SiO, where R is a hydrogen atom or a hydrocarbon group. They belong to the wider class of organosilicon compounds....
, and lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s.
Polar monomers, using controlled conditions and low temperatures, can undergo anionic polymerization. However, at higher temperatures they do not produce living stable, carbanionic chain ends because their polar substituents can undergo side reactions with both initiators and propagating chain centers. The effects of counterion, solvent, temperature, Lewis base additives, and inorganic solvents have been investigated to increase the potential of anionic polymerizations of polar monomers. Polar monomers include acrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
, cyanoacrylate
Cyanoacrylate
Cyanoacrylate is the generic name for cyanoacrylate based fast-acting adhesives such as methyl 2-cyanoacrylate, ethyl-2-cyanoacrylate , and n-butyl cyanoacrylate...
, propylene oxide
Propylene oxide
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics...
, vinyl ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
, acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
, vinyl sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
, vinyl sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
, vinyl silane and isocyanate
Isocyanate
Isocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
.

Solvent
The solvent used in anionic addition polymerizations are determined by the reactivity of both the initiator and carbanion of the propagating chain end.The stability of the anionic propagating species is also dependent on the solvent as it is significantly reduced in polar solvents such as ethers due to the presence of the nucleophilic C-O bond of the ether. Less reactive chain ends, such as heterocyclic monomers, can use a wide range of solvents.Initiation
The reactivity of initiators used in anionic polymerization should be similar to that of the monomer that is the propagating species. The pKa values for the conjugate acids of the carbanions formed from monomers can be used to deduce the reactivity of the monomer. The least reactive monomers have the largest pKa values for their corresponding conjugate acid and thus, require the most reactive initiator. Two main initiation pathways involve electron transfer (through alkali metals) and strong anions.Initiation by Electron Transfer
Szwarc and coworkers studied the initiation of polymerization through the use of aromatic radical-anions such as sodium naphthenate. In this reaction, an electron is transferred from the alkali metal to naphthaleneNaphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
. Polar solvents are necessary for this type of initiation both for stability of the anion-radical and to solvate the cation species formed. The anion-radical can then transfer an electron to the monomer.

Initiation by Strong Anions
Nucleophilic initiators include covalent or ionic metal amideAmide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s, alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
s, hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
s, cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
s, phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
s, amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s and organometallic compounds (alkyllithium compounds and Grignard reagents). The initiation process involves the addition of a neutral (B:) or negative (B:-) nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
to the monomer.

Propagation
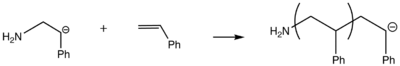
Termination
Anionic addition polymerizations have no formal termination pathways because proton transfer from solvent or other positive species does not occur. However, termination can occur through unintentional quenching due to trace impurities. This includes trace amounts of oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
or water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. Intentional termination can occur through the addition of water or alcohol. Another method of termination, chain transfer, can occur when an agent can act as a Brønsted acid. In this case, the pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
value of the agent is similar to the conjugate acid of the propagating carbanionic chain end. Spontaneous termination occurs because the concentration of carbanion centers decay over time and eventually results in hydride elimination.
Polar monomers are more reactive because they are stabilized by their polar substituents. These polar substituents can react with nucleophiles which results in termination as well as side reactions that compete with both initiation and propagation.
Living Anionic Polymerization
Living polymerization was demonstrated by Szwarc and co workers in 1956. Their initial work was based on the polymerization of styrene and dienes.One of the remarkable features of living anionic polymerization is that the mechanism involves no formal termination step. In the absence of impurities, the carbanion would still be active and capable of adding another monomer. The chains will remain active indefinitely unless there is inadvertent or deliberate termination or chain transfer.
Kinetics
The kineticsChemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
of anionic addition polymerization depend on whether or not a termination pathway occurs.
Kinetics of Living Anionic Addition Polymerization
In general, the reaction mechanism for living anionic addition polymerization are as follows:-

where I = initiator, kinit = the initiation reaction rate constant, M = monomer, M-= propagating species, and kprop = the propagation reaction rate constant.
As most polymerizations of this type do not have a termination pathway, the rate of polymerization is the rate of propagation:
where kp is the rate of constant of propagation, [M-] is the total concentration of propagating centers, and [M] is the concentration of monomer.
Since there is no termination pathway in living polymerizations, the concentration of propagating centers is equal to the concentration of initiator ([I]).
Thus,
The degree of polymerizationDegree of polymerizationThe degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.For a homopolymer, there is only one type of monomeric unit andthe number-average degree of polymerization is given by...
, Xn is also affected by no termination pathway. It is the ratio of concentration of reacted monomer ([M]o) to initiator([I]o) times the percent conversion p. In this case, the chain length (ν) is equal to Xn.
When conversion, p = 1 (100% conversion), chain length is simply the ratio of reacted monomer to initiator.
Kinetics: Termination due to Impurities
When termination occurs due to impurities, the impurities must be taken into account in determining the reaction rate.
The reaction mechanisms would begin the same as that of a living anionic addition (initiation and propagation). However, there would now be a termination step to account for the effect of the impurities on the reaction.
where M-= propagating species, HX = impurity and kterm = the termination reaction rate constant.
Using the steady-state approximation, the rate of propagation becomes
Since
Thus chain length and rate of propagation are negatively impacted by the presence of impurities in the reaction. -







