
Sulfoxide
Encyclopedia
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
containing a sulfinyl functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides
Thioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
. (The use of the alternative spelling sulphoxide is discouraged by IUPAC.) An example of a sulfoxide occurring in nature is alliin
Alliin
Alliin is a sulfoxide that is a natural constituent of fresh garlic. It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic...
.
Nature of the bond
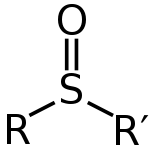
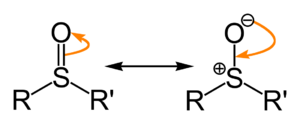
Sulfoxides are generally represented with the structural formula R–S(=O)–R', where R and R' are organic groups. The bond between the sulfurSulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atoms differs from the conventional double bond between carbon and oxygen in, say, ketones. The S–O interaction has an electrostatic aspect, resulting in significant dipolar
Bond dipole moment
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
character, with negative charge centered on oxygen. The bonding is similar to that in tertiary phosphine oxides, R3P=O.
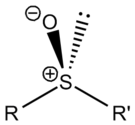
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
of electrons resides on the sulfur atom giving it tetrahedral molecular geometry
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
as for sp³ carbon. When the two organic residues are dissimilar, the sulfur is a chiral center
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
, for example, methylphenylsulfoxide.
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
is sufficiently high that sulfoxides are optically stable, that is, the rate of racemization
Racemization
In chemistry, racemization refers to the converting of an enantiomerically pure mixture into a mixture where more than one of the enantiomers are present...
is slow at room temperature. Chiral sulfoxides find application in certain drugs such as esomeprazole
Esomeprazole
Esomeprazole is a proton pump inhibitor developed and marketed by AstraZeneca which is used in the treatment of dyspepsia, peptic ulcer disease , gastroesophageal reflux disease and Zollinger-Ellison syndrome...
and Armodafinil
Armodafinil
Armodafinil is a stimulant-like drug produced by the pharmaceutical company Cephalon Inc., which was approved by the FDA on June 15, 2007...
, and they are also employed as chiral auxiliaries
Chiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
. Many chiral sulfoxides are prepared from asymmetric catalytic oxidation
Asymmetric catalytic oxidation
Asymmetric catalytic oxidation is a technique of oxidizing various substrates to give an enantiopure product using a catalyst.-Reactions:*Jacobsen epoxidation of alkenes using manganese-salen complex and NaOCl...
of achiral sulfides with a transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
and a chiral ligand.
Reactions
SulfideSulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
s are the usual starting materials to sulfoxides by organic oxidation. For example, dimethyl sulfide
Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
with oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of -2 is oxidized to dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
with oxidation state 0. Further oxidation converts the compound to dimethyl sulfone
Methylsulfonylmethane
Methylsulfonylmethane is an organosulfur compound with the formula 2SO2. It is also known by several other names including DMSO2, methyl sulfone, and dimethyl sulfone. This colorless solid features the sulfonyl functional group and is considered relatively inert chemically...
wherein sulfur has the oxidation state +2. They are also excellent directing groups.
Sulfoxides, such as DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, have basic character, being excellent ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s and readily alkylated. Alkyl sulfoxides are susceptible to deprotonation by strong bases, such as sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
.

