Wallach rearrangement
Encyclopedia
The Wallach rearrangement, named after Otto Wallach
, is an organic reaction
and a rearrangement reaction
converting an aromatic azoxy compound
with sulfuric acid
to an azo compound
with one arene ring substituted
by an hydroxyl
group in the aromatic para position.
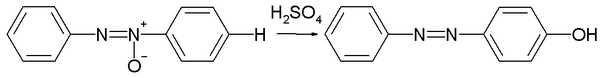 60% to 100% sulfuric acid is required.
60% to 100% sulfuric acid is required.
Conceptually related reactions are the Fries rearrangement
, the Fischer-Hepp rearrangement
, the Bamberger rearrangement
, the benzidine rearrangement and the Hofmann-Martius rearrangement
.
for this reaction is not known with great precision despite experimental evidence:
A mechanism not inconsistent with these findings is depicted below:
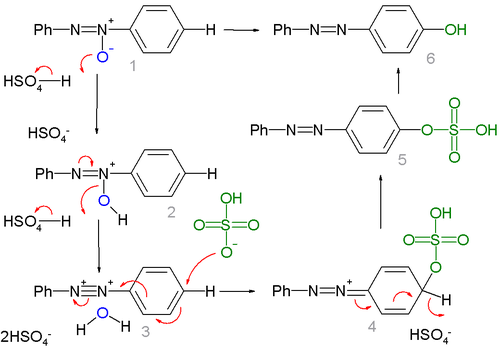 NOTE The curly arrows in intermediate 4 go in the wrong direction.
NOTE The curly arrows in intermediate 4 go in the wrong direction.
In the first part of the reaction two equivalents of acid tease the oxygen atom away from the azoxy group. The resulting dicationic intermediate 3 with an unusual R-N+:::N+-R motif in this scheme has been observed by proton NMR
in a system of fluoroantimonic acid
and azoxybenzene at -50°C. In the second part of the reaction the HSO4- anion is a nucleophile
in a nucleophilic aromatic substitution
to 5 followed by hydrolysis
to 6.
Otto Wallach
Otto Wallach was a German chemist and recipient of the 1910 Nobel prize in Chemistry for his work on alicyclic compounds.-Biography:...
, is an organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
and a rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
converting an aromatic azoxy compound
Azoxy
Azoxy compounds are a group of chemical compounds sharing a common functional group with the general structure RN=N+R. They are considered N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles. They give 1,3 dipolar cycloaddition with double bonds....
with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
to an azo compound
Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
with one arene ring substituted
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
by an hydroxyl
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
group in the aromatic para position.

Conceptually related reactions are the Fries rearrangement
Fries rearrangement
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenyl ester to a hydroxy aryl ketone by catalysis of Lewis acids.It involves migration of an acyl group of phenyl ester to benzene ring.- Mechanism:...
, the Fischer-Hepp rearrangement
Fischer-Hepp rearrangement
The Fischer-Hepp rearrangement is a rearrangement reaction in which an aromatic N-nitroso or nitrosamine converts to a carbon nitroso compound:This organic reaction was first described by the German chemist Otto Philipp Fischer and...
, the Bamberger rearrangement
Bamberger rearrangement
The Bamberger rearrangement is the chemical reaction of N-phenylhydroxylamines with strong aqueous acid, which will rearrange to give 4-aminophenols...
, the benzidine rearrangement and the Hofmann-Martius rearrangement
Hofmann-Martius rearrangement
The Hofmann–Martius rearrangement in organic chemistry is a rearrangement reaction converting an N-alkylated aniline to the corresponding ortho and / or para aryl-alkylated aniline...
.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for this reaction is not known with great precision despite experimental evidence:
- The primary kinetic isotope effectKinetic isotope effectThe kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
for the arene proton is close to one excluding the corresponding C-H bond from breaking in the rate-determining stepRate-determining stepThe rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In... - The chemical kineticsChemical kineticsChemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
of the reaction point to involvement of two protons in the reaction: the reaction rateReaction rateThe reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of the rearrangement continues to increase beyond the stage of complete monoprotonation of the substrate. - Other kinetic evidence identifies the second proton donation as the rate-determining stepRate-determining stepThe rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
- The phenolic oxygen atom in the product is not the oxygen atom in the reactant but provided by solventSolventA solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, based on isotopic scrambling experiments. - Furthermore isotope labeling of the N-O nitrogen atom in azoxybenzene gives the azo compound with the 15N isotope distributed over both nitrogen atoms indicating a symmetrical intermediate.
A mechanism not inconsistent with these findings is depicted below:

In the first part of the reaction two equivalents of acid tease the oxygen atom away from the azoxy group. The resulting dicationic intermediate 3 with an unusual R-N+:::N+-R motif in this scheme has been observed by proton NMR
Proton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
in a system of fluoroantimonic acid
Fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride in various ratios. The 1:1 combination forms the strongest known superacid, which has been demonstrated to protonate even hydrocarbons to afford carbocations and H2....
and azoxybenzene at -50°C. In the second part of the reaction the HSO4- anion is a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
in a nucleophilic aromatic substitution
Nucleophilic aromatic substitution
right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
to 5 followed by hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
to 6.

