Fluoroantimonic acid
Encyclopedia
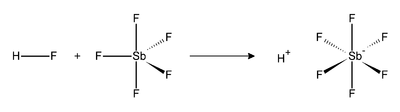
Fluoroantimonic acid is a mixture of hydrogen fluoride
and antimony pentafluoride
in various ratios. The 1:1 combination forms the strongest known superacid
, which has been demonstrated to protonate even hydrocarbon
s to afford carbocation
s and H2.
The reaction of hydrogen fluoride (HF) and SbF5 is exothermic. HF, being a Lewis base, attacks the molecules of SbF5 to give an adduct. In the fluoroantimonic molecule, the anion is coordinated to the hydrogen, although the anion is formally classified as noncoordinating, because it is both a very weak nucleophile
and a very weak base
.
Despite the proton
being called effectively "naked," it is in fact always attached to a fluorine through a very weak dative bond, similar to the hydronium
cation. However, the weakness of this bond accounts for the system's extreme acidity. Fluoroantimonic acid is 2×1019 (20 quintillion) times stronger than 100% sulfuric acid
.
. These salts have the formulas [H2F+][Sb2F11−] and [H3F2+][Sb2F11−]. In both salts the anion is Sb2F11−. As mentioned above, SbF6− is classified as weakly basic; the larger monoanion Sb2F11− would be expected to be still weaker.
. Acidity is indicated by large negative values of H0.
s. In 1967, Bickel and Hogeveen showed that HF-SbF5 will remove H2 from isobutane
and methane from neopentane
:3CH + H+ → (CH3)3C+ + H24C + H+ → (CH3)3C+ + CH4
s that have been proven to be compatible with HF-SbF5 are SO2ClF
and liquefied sulfur dioxide
. Chlorofluorocarbon
s have also been used as solvents. Containers for
HF-SbF5 are made of PTFE
.
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
and antimony pentafluoride
Antimony pentafluoride
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a valuable Lewis acid and a component of the superacid fluoroantimonic acid, the strongest known acid...
in various ratios. The 1:1 combination forms the strongest known superacid
Superacid
According to the classical definition superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function of −12. According to the modern definition, superacid is a medium, in which the chemical potential of the proton is higher than in pure...
, which has been demonstrated to protonate even hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s to afford carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s and H2.
The reaction of hydrogen fluoride (HF) and SbF5 is exothermic. HF, being a Lewis base, attacks the molecules of SbF5 to give an adduct. In the fluoroantimonic molecule, the anion is coordinated to the hydrogen, although the anion is formally classified as noncoordinating, because it is both a very weak nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
and a very weak base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
.
Despite the proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
being called effectively "naked," it is in fact always attached to a fluorine through a very weak dative bond, similar to the hydronium
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
cation. However, the weakness of this bond accounts for the system's extreme acidity. Fluoroantimonic acid is 2×1019 (20 quintillion) times stronger than 100% sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
.
Structure
Two related products have been crystallised from HF-SbF5 mixtures, and both have been analyzed by single crystal X-ray crystallographyCrystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
. These salts have the formulas [H2F+][Sb2F11−] and [H3F2+][Sb2F11−]. In both salts the anion is Sb2F11−. As mentioned above, SbF6− is classified as weakly basic; the larger monoanion Sb2F11− would be expected to be still weaker.
Comparison with other acids
The following values are based upon the Hammett acidity functionHammett acidity function
The Hammett acidity function is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity function used to extend the measure of acidity beyond the...
. Acidity is indicated by large negative values of H0.
- Fluoroantimonic acid (1990) (H0 Value = −31.3)
- Magic acidMagic acidMagic acid , is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfonic acid and antimony pentafluoride...
(1974) (H0 Value = −19.2) - Carborane superacid (1969) (H0 Value = −18.0)
- Fluorosulfuric acidFluorosulfuric acidFluorosulfuric acid is the inorganic compound with the formula HSO3F. It is one of the strongest acids commercially available and is a superacid. The formula HFSO3 emphasizes its relationship to sulfuric acid, H2SO4; HSO3F is a tetrahedral molecule.-Chemical properties:Fluorosulfuric acid is a...
(1944) (H0 Value = −15.1) - Triflic acid (1940) (H0 Value = −14.9)
Applications
This extraordinarily strong acid protonates nearly all organic compoundOrganic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s. In 1967, Bickel and Hogeveen showed that HF-SbF5 will remove H2 from isobutane
Isobutane
Isobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,...
and methane from neopentane
Neopentane
Neopentane, also called dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is an extremely flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher...
:3CH + H+ → (CH3)3C+ + H24C + H+ → (CH3)3C+ + CH4
Safety
HF-SbF5 is rapidly and explosively decomposed by water. It reacts with virtually all known solvents. SolventSolvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s that have been proven to be compatible with HF-SbF5 are SO2ClF
Sulfuryl chloride fluoride
Sulfuryl chloride fluoride is the chemical compound with the formula SO2ClF. It is employed as a solvent for highly oxidizing compounds.The laboratory-scale synthesis begins with the preparation of potassium fluorosulfite:This salt is then chlorinated to give sulfuryl chloride fluorideFurther...
and liquefied sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
. Chlorofluorocarbon
Chlorofluorocarbon
A chlorofluorocarbon is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons , which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon...
s have also been used as solvents. Containers for
HF-SbF5 are made of PTFE
Polytetrafluoroethylene
Polytetrafluoroethylene is a synthetic fluoropolymer of tetrafluoroethylene that finds numerous applications. PTFE is most well known by the DuPont brand name Teflon....
.