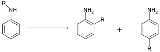
Hofmann-Martius rearrangement
Encyclopedia
The Hofmann–Martius rearrangement in organic chemistry
is a rearrangement reaction
converting an N-alkylated aniline
to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat and the catalyst is an acid like hydrochloric acid
When the catalyst is a metal halide the reaction is also called the Reilly–Hickinbottom rearrangement.
The reaction is also known to work for aryl ethers and two conceptually related reactions are the Fries rearrangement
and the Fischer–Hepp rearrangement. Its reaction mechanism
centers around dissociation of the reactant with the positively charged organic residue R attacking the aniline ring in a Friedel–Crafts alkylation.
In one study this rearrangement is applied to a 3-N(CH3)(C6H5)-2-oxindole :
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
converting an N-alkylated aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat and the catalyst is an acid like hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
When the catalyst is a metal halide the reaction is also called the Reilly–Hickinbottom rearrangement.
The reaction is also known to work for aryl ethers and two conceptually related reactions are the Fries rearrangement
Fries rearrangement
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenyl ester to a hydroxy aryl ketone by catalysis of Lewis acids.It involves migration of an acyl group of phenyl ester to benzene ring.- Mechanism:...
and the Fischer–Hepp rearrangement. Its reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
centers around dissociation of the reactant with the positively charged organic residue R attacking the aniline ring in a Friedel–Crafts alkylation.
In one study this rearrangement is applied to a 3-N(CH3)(C6H5)-2-oxindole :



