
Membrane transport
Encyclopedia
In cellular biology the term membrane transport refers to the collection of mechanisms that regulate the passage of solutes such as ions and small molecules through biological membranes namely lipid bilayers that contain proteins embedded in them. The regulation of passage through the membrane is due to selective membrane permeability - a characteristic of biological membranes which allows them to separate substances of distinct chemical nature. In other words, they can be permeable to certain substances but not to others.
The movements of most solutes through the membrane are mediated by membrane transport proteins which are specialized to varying degrees in the transport of specific molecules. As the diversity and physiology
of the distinct cells
is highly related to their capacities to attract different external elements, it is postulated that there is a group of specific transport proteins for each cell type and for every specific physiological stage[1]. This differential expression is regulated through the differential transcription
of the genes
coding for these proteins and its translation, for instance, through genetic-molecular mechanisms, but also at the cell biology level: the production of these proteins can be activated by cellular signaling pathways, at the biochemical level, or even by being situated in cytoplasmic vesicles.
or electrochemical gradient
or against it. If the exchange of substances occurs in the direction of the gradient, that is, in the direction of decreasing potential, there is no requirement for an input of energy from outside the system; if, however, the transport is against the gradient, it will require the input of energy, metabolic energy in this case.
For example, a classic chemical mechanism for separation that does not require the addition of external energy is dialysis. In this system a semipermeable membrane separates two solutions of different concentration of the same solute. If the membrane allows the passage of water but not the solute the water will move into the compartment with the greatest solute concentration in order to establish an equilibrium in which the energy of the system is at a minimum. This takes place because the water moves from a high solvent concentration to a low one (in terms of the solute, the opposite occurs) and because the water is moving along a gradient there is no need for an external input of energy.
The nature of biological membranes, especially that of its lipids, is amphiphilic, as they form bilayers that contain an internal hydrophobic layer and an external hydrophilic layer. This structure makes transport possible by simple or passive diffusion, which consists of the diffusion
of substances through the membrane without expending metabolic energy and without the aid of transport proteins. If the transported substance has a net electrical charge, it will move not only in response to a concentration gradient, but also to an electrochemical gradient
due to the membrane potential
.
As few molecules are able to diffuse through a lipid membrane the majority of the transport processes involve transport proteins. These transmembrane proteins possess a large number of alpha helices immersed in the lipid matrix. In bacteria these proteins are present in the beta lamina form. This structure probably involves a conduit through hydrophilic protein environments that cause a disruption in the highly hydrophobic medium formed by the lipids.[1] These proteins can be involved in transport in a number of ways: they act as pumps driven by ATP
, that is, by metabolic energy, or as channels of facilitated diffusion.
A general principle of thermodynamics that governs the transfer of substances through membranes and other surfaces is that the exchange of free energy
, ΔG, for the transport of a mole
of a substance of concentration C1 in a compartment to another compartment where it is present at C2 is:
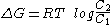
Where C2 is less than C1 ΔG is negative, and the process is thermodynamically favorable. As the energy is transferred from one compartment to another, except where other factors intervene, an equilibrium will be reached where C2=C1, and where G=0. However, there are three circumstances under which this equilibrium will not be reached, circumstances which are vital for the in vivo functioning of biological membranes:
Where F is Faraday's constant and ΔP the membrane potential in volts. If ΔP is negative and Z is positive, the contribution of the term ZFΔP to ΔG will be negative, that is, it will favor the transport of cations from the interior of the cell. So, if the potential difference is maintained, the equilibrium state ΔG=0 will not correspond to a equimolar concentration of ions on both sides of the membrane.
Where ΔGb corresponds to a favorable thermodynamic reaction, such as the hydrolysis of ATP, or the co-transport
of a compound that is moved in the direction of its gradient.
of a system and decreases the free energy. The transport process is influenced by the characteristics of the transport substance and the nature of the bilayer. Membrane proteins are not involved in passive diffusion. The diffusion velocity of a pure phospholipid membrane will depend on:
the hydrolysis of the energy provider (e.g. ATP) takes place directly in order to transport the solute in question, for instance, when the transport proteins are ATPase
enzymes. Where the hydrolysis of the energy provider is indirect as is the case in secondary active transport
, use is made of the energy stored in an electrochemical gradient. For example, in co-transport
use is made of the gradients of certain solutes to transport a target compound against its gradient, causing the dissipation of the solute gradient. It may appear that, in this example, there is no energy use, but hydrolysis of the energy provider is required to establish the gradient of the solute transported along with the target compound. The gradient of the co-transport
ed solute will be generated through the use of certain types of proteins called biochemical pumps.
The discovery of the existence of this type of transporter protein came from the study of the kinetics of cross-membrane molecule transport. For certain solutes it was noted that the transport velocity reached a plateau at a particular concentration above which there was no significant increase in uptake rate, indicating a log curve type response. This was interpreted as showing that transport was mediated by the formation of a substrate-transporter complex, which is conceptually the same as the enzyme-substrate complex of enzyme kinetics
. Therefore, each transport protein has an affinity constant for a solute that is equal to the concentration of the solute when the transport velocity is half its maximum value. This is equivalent in the case of an enzyme to the Michaelis-Menten constant.
Some important features of active transport in addition to its ability to intervene even against a gradient, its kinetics and the use of ATP, are its high selectivity and ease of selective pharmacological inhibition
Both can be referred to as co-transporters.
characteristics on it. This gradient is of interest as an indicator of the state of the cell through parameters such as the Nernst potential. In terms of membrane transport the gradient is of interest as it contributes to increased system entropy in the co-transport
of substances against their gradient.
One of the most important pumps in animal cells is the sodium potassium pump, that operates through the following mechanism:
and the interaction of the ion with the internal charges of the pore.
In order for an ion to pass through a pore it must dissociate itself from the water molecules that cover it in successive layers of solvation
. The tendency to dehydrate, or the facility to do this, is related to the size of the ion: larger ions can do it more easily that the smaller ions, so that a pore with weak polar centres will preferentially allow passage of larger ions over the smaller ones.
When the interior of the channel is composed of polar groups from the side chains of the component amino acids, the interaction of a dehydrated ion with these centres can be more important than the facility for dehydration in conferring the specificity of the channel. For example, a channel made up of histidines and arginines, with positively charged groups, will selectively repel ions of the same polarity, but will facilitate the passage of negatively charged ions. Also, in this case, the smallest ions will be able to interact more closely due to the spatial arrangement of the molecule (stericity), which greatly increases the charge-charge interactions and therefore exaggerates the effect.
Partially charged non-electrolytes, that are more or less polar, such as ethanol, methanol or urea, are able to pass through the membrane through aqueous channels immersed in the membrane. It is interesting to note that there is no effective regulation mechanism that limits this transport, which indicates an intrinsic vulnerability of the cells to the penetration of these molecules.
The movements of most solutes through the membrane are mediated by membrane transport proteins which are specialized to varying degrees in the transport of specific molecules. As the diversity and physiology
Physiology
Physiology is the science of the function of living systems. This includes how organisms, organ systems, organs, cells, and bio-molecules carry out the chemical or physical functions that exist in a living system. The highest honor awarded in physiology is the Nobel Prize in Physiology or...
of the distinct cells
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
is highly related to their capacities to attract different external elements, it is postulated that there is a group of specific transport proteins for each cell type and for every specific physiological stage[1]. This differential expression is regulated through the differential transcription
Transcription (genetics)
Transcription is the process of creating a complementary RNA copy of a sequence of DNA. Both RNA and DNA are nucleic acids, which use base pairs of nucleotides as a complementary language that can be converted back and forth from DNA to RNA by the action of the correct enzymes...
of the genes
Gênes
Gênes is the name of a département of the First French Empire in present Italy, named after the city of Genoa. It was formed in 1805, when Napoleon Bonaparte occupied the Republic of Genoa. Its capital was Genoa, and it was divided in the arrondissements of Genoa, Bobbio, Novi Ligure, Tortona and...
coding for these proteins and its translation, for instance, through genetic-molecular mechanisms, but also at the cell biology level: the production of these proteins can be activated by cellular signaling pathways, at the biochemical level, or even by being situated in cytoplasmic vesicles.
Background
Thermodynamically the flow of substances from one compartment to another can occur in the direction of a concentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
or electrochemical gradient
Gradient
In vector calculus, the gradient of a scalar field is a vector field that points in the direction of the greatest rate of increase of the scalar field, and whose magnitude is the greatest rate of change....
or against it. If the exchange of substances occurs in the direction of the gradient, that is, in the direction of decreasing potential, there is no requirement for an input of energy from outside the system; if, however, the transport is against the gradient, it will require the input of energy, metabolic energy in this case.
For example, a classic chemical mechanism for separation that does not require the addition of external energy is dialysis. In this system a semipermeable membrane separates two solutions of different concentration of the same solute. If the membrane allows the passage of water but not the solute the water will move into the compartment with the greatest solute concentration in order to establish an equilibrium in which the energy of the system is at a minimum. This takes place because the water moves from a high solvent concentration to a low one (in terms of the solute, the opposite occurs) and because the water is moving along a gradient there is no need for an external input of energy.
The nature of biological membranes, especially that of its lipids, is amphiphilic, as they form bilayers that contain an internal hydrophobic layer and an external hydrophilic layer. This structure makes transport possible by simple or passive diffusion, which consists of the diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of substances through the membrane without expending metabolic energy and without the aid of transport proteins. If the transported substance has a net electrical charge, it will move not only in response to a concentration gradient, but also to an electrochemical gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
due to the membrane potential
Membrane potential
Membrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
.
| Type of substance | Examples | Behaviour |
|---|---|---|
| Gases | CO2, N2, O2 | Permeable |
| Small uncharged polar molecules | Urea Urea Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group.... , water Water Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a... , ethanol Ethanol Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a... |
Permeable, totally or partially |
| Large uncharged polar molecules | glucose Glucose Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate... , fructose Fructose Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847... |
Not permeable |
| Ions | K+, Na+, Cl-, HCO3- | Not permeable |
| Charged polar molecules | ATP Adenosine triphosphate Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism... , amino acid Amino acid Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen... s, glucose-6-phosphate Glucose-6-phosphate Glucose 6-phosphate is glucose sugar phosphorylated on carbon 6. This compound is very common in cells as the vast majority of glucose entering a cell will become phosphorylated in this way.... |
Not permeable |
As few molecules are able to diffuse through a lipid membrane the majority of the transport processes involve transport proteins. These transmembrane proteins possess a large number of alpha helices immersed in the lipid matrix. In bacteria these proteins are present in the beta lamina form. This structure probably involves a conduit through hydrophilic protein environments that cause a disruption in the highly hydrophobic medium formed by the lipids.[1] These proteins can be involved in transport in a number of ways: they act as pumps driven by ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, that is, by metabolic energy, or as channels of facilitated diffusion.
Thermodynamics
A physiological process can only take place if it complies with basic thermodynamic principles. Membrane transport obeys physical laws that define its capabilities and therefore its biological utility.A general principle of thermodynamics that governs the transfer of substances through membranes and other surfaces is that the exchange of free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
, ΔG, for the transport of a mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of a substance of concentration C1 in a compartment to another compartment where it is present at C2 is:
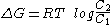
Where C2 is less than C1 ΔG is negative, and the process is thermodynamically favorable. As the energy is transferred from one compartment to another, except where other factors intervene, an equilibrium will be reached where C2=C1, and where G=0. However, there are three circumstances under which this equilibrium will not be reached, circumstances which are vital for the in vivo functioning of biological membranes:
- The macromolecules on one side of the membrane can bond preferentially to a certain component of the membrane or chemically modify it. In this way, although the concentration of the solute may actually be different on both sides of the membrane, the availability of the solute is reduced in one of the compartments to such an extent that, for practical purposes, no gradient exists to drive transport.
- A membrane electrical potentialMembrane potentialMembrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
can exist which can influence ion distribution. For example, for the transport of ions from the exterior to the interior, it is possible that: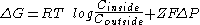
Where F is Faraday's constant and ΔP the membrane potential in volts. If ΔP is negative and Z is positive, the contribution of the term ZFΔP to ΔG will be negative, that is, it will favor the transport of cations from the interior of the cell. So, if the potential difference is maintained, the equilibrium state ΔG=0 will not correspond to a equimolar concentration of ions on both sides of the membrane.
- If a process with a negative ΔG is coupled to the transport process then the global ΔG will be modified. This situation is common in active transport and is described thus:
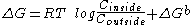
Where ΔGb corresponds to a favorable thermodynamic reaction, such as the hydrolysis of ATP, or the co-transport
Co-transport
Co-transport, also known as coupled transport or secondary active transport, refers to the simultaneous or sequential passive transfer of molecules or ions across biological membranes in a fixed ratio...
of a compound that is moved in the direction of its gradient.
Passive diffusion
As mentioned above, passive diffusion is a spontaneous phenomenon that increases the entropyEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
of a system and decreases the free energy. The transport process is influenced by the characteristics of the transport substance and the nature of the bilayer. Membrane proteins are not involved in passive diffusion. The diffusion velocity of a pure phospholipid membrane will depend on:
- concentration gradient,
- hydrophobicity,
- size,
- charge, if the molecule has a net charge.
Active transport and co-transport
In active transport a solute is moved against a concentration or electrochemical gradient, in doing so the transport proteins involved consume metabolic energy, usually ATP. In primary active transportPrimary active transport
Primary active transport, also called direct active transport, directly uses energy to transport molecules across a membrane.Most of the enzymes that perform this type of active transport are transmembrane ATPases. A primary ATPase universal to all cellular life is the sodium-potassium pump, which...
the hydrolysis of the energy provider (e.g. ATP) takes place directly in order to transport the solute in question, for instance, when the transport proteins are ATPase
ATPase
ATPases are a class of enzymes that catalyze the decomposition of adenosine triphosphate into adenosine diphosphate and a free phosphate ion. This dephosphorylation reaction releases energy, which the enzyme harnesses to drive other chemical reactions that would not otherwise occur...
enzymes. Where the hydrolysis of the energy provider is indirect as is the case in secondary active transport
Secondary active transport
In secondary active transport or co-transport, uses energy to transport molecules across a membrane; however, in contrast to primary active transport, there is no direct coupling of ATP; instead, the electrochemical potential difference created by pumping ions out of the cell is used...
, use is made of the energy stored in an electrochemical gradient. For example, in co-transport
Co-transport
Co-transport, also known as coupled transport or secondary active transport, refers to the simultaneous or sequential passive transfer of molecules or ions across biological membranes in a fixed ratio...
use is made of the gradients of certain solutes to transport a target compound against its gradient, causing the dissipation of the solute gradient. It may appear that, in this example, there is no energy use, but hydrolysis of the energy provider is required to establish the gradient of the solute transported along with the target compound. The gradient of the co-transport
Co-transport
Co-transport, also known as coupled transport or secondary active transport, refers to the simultaneous or sequential passive transfer of molecules or ions across biological membranes in a fixed ratio...
ed solute will be generated through the use of certain types of proteins called biochemical pumps.
The discovery of the existence of this type of transporter protein came from the study of the kinetics of cross-membrane molecule transport. For certain solutes it was noted that the transport velocity reached a plateau at a particular concentration above which there was no significant increase in uptake rate, indicating a log curve type response. This was interpreted as showing that transport was mediated by the formation of a substrate-transporter complex, which is conceptually the same as the enzyme-substrate complex of enzyme kinetics
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
. Therefore, each transport protein has an affinity constant for a solute that is equal to the concentration of the solute when the transport velocity is half its maximum value. This is equivalent in the case of an enzyme to the Michaelis-Menten constant.
Some important features of active transport in addition to its ability to intervene even against a gradient, its kinetics and the use of ATP, are its high selectivity and ease of selective pharmacological inhibition
Transporter proteins
A transport protein can move various ions and molecules, they are distinguished according to their directionality:- antiporterAntiporterAn antiporter is an integral membrane protein involved in secondary active transport of two or more different molecules or ions across a phospholipid membrane such as the plasma membrane in opposite directions.In secondary active transport, one species of solute moves along its electrochemical...
: (also called exchanger or counter-transporter) transport proteins which transport a molecule against its gradient and at the same time displaces one or more ions along its gradient, both gradients being opposite, - symporterSymporterA cotransporter is an integral membrane protein that is involved in secondary active transport. It works by binding to two molecules or ions at a time and using the gradient of one solute's concentration to force the other molecule or ion against its gradient....
: transport proteins which move a molecule against its gradient while displacing one or more different ions along their gradient which is in the same direction as that of the transported molecule.
Both can be referred to as co-transporters.
Pumps
A pump is a protein that hydrolyses ATP in order to transport a particular solute through a membrane in order to generate an electrochemical gradient to confer certain membrane potentialMembrane potential
Membrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
characteristics on it. This gradient is of interest as an indicator of the state of the cell through parameters such as the Nernst potential. In terms of membrane transport the gradient is of interest as it contributes to increased system entropy in the co-transport
Co-transport
Co-transport, also known as coupled transport or secondary active transport, refers to the simultaneous or sequential passive transfer of molecules or ions across biological membranes in a fixed ratio...
of substances against their gradient.
One of the most important pumps in animal cells is the sodium potassium pump, that operates through the following mechanism:
- binding of three Na+ ions to their active sites on the pump which are bound to ATP.
- ATP is hydrolyzed leading to phosphorylation of the cytoplasmic side of the pump, this induces a structure change in the protein. The phosphorylation is caused by the transfer of the terminal group of ATP to a residue of aspartate in the transport protein and the subsequent release of ADP.
- the structure change in the pump exposes the Na+ to the exterior. The phosphorylated form of the pump has a low affinity for Na+ ions so they are released.
- once the Na+ ions are liberated, the pump binds two molecules of K+ to their respective bonding sites on the extracellular face of the transport protein.This causes the dephosphorylation of the pump, reverting it to its previous conformational state, transporting the K+ ions into the cell.
- The unphosphorylated form of the pump has a higher affinity for Na+ ions than K+ ions, so the two bound K+ ions are released into the cytosolCytosolThe cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
. ATP binds, and the process starts again.
Membrane selectivity
As the main characteristic of transport through a biological membrane is its selectivity and its subsequent behavior as a barrier for certain substances, the underlying physiology of the phenomenon has been studied extensively. Investigation into membrane selectivity have classically been divided into those relating to electrolytes and non-electrolytes.Electrolyte selectivity
The ionic channels define an internal diameter that permits the passage of small ions that is related to various characteristics of the ions that could potentially be transported. As the size of the ion is related to its chemical species, it could be assumed a priori that a channel whose pore diameter was sufficient to allow the passage of one ion would also allow the transfer of others of smaller size, however, this does not occur in the majority of cases. There are two characteristics alongside size that are important in the determination of the selectivity of the membrane pores: the facility for dehydrationDehydration
In physiology and medicine, dehydration is defined as the excessive loss of body fluid. It is literally the removal of water from an object; however, in physiological terms, it entails a deficiency of fluid within an organism...
and the interaction of the ion with the internal charges of the pore.
In order for an ion to pass through a pore it must dissociate itself from the water molecules that cover it in successive layers of solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
. The tendency to dehydrate, or the facility to do this, is related to the size of the ion: larger ions can do it more easily that the smaller ions, so that a pore with weak polar centres will preferentially allow passage of larger ions over the smaller ones.
When the interior of the channel is composed of polar groups from the side chains of the component amino acids, the interaction of a dehydrated ion with these centres can be more important than the facility for dehydration in conferring the specificity of the channel. For example, a channel made up of histidines and arginines, with positively charged groups, will selectively repel ions of the same polarity, but will facilitate the passage of negatively charged ions. Also, in this case, the smallest ions will be able to interact more closely due to the spatial arrangement of the molecule (stericity), which greatly increases the charge-charge interactions and therefore exaggerates the effect.
Non-electrolyte selectivity
Non-electrolytes, substances that generally are hydrophobic and lipophylic, usually pass through the membrane by dissolution in the lipid bilayer, and therefore, by passive diffusion. For those non-electrolytes whose transport through the membrane is mediated by a transport protein the ability to diffuse is, generally, dependent on the partition coefficient K.Partially charged non-electrolytes, that are more or less polar, such as ethanol, methanol or urea, are able to pass through the membrane through aqueous channels immersed in the membrane. It is interesting to note that there is no effective regulation mechanism that limits this transport, which indicates an intrinsic vulnerability of the cells to the penetration of these molecules.
Evolution of Membrane Transport Proteins
There are several databases which attempt to construct phylogenetic trees detailing the evolution of transporter proteins. One such resource is the Transporter Classification databaseSee also
- Passive diffusion through the cell membrane
- Cellular transport
- Trans-membrane transport

