
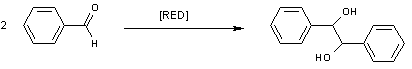
Pinacol coupling reaction
Encyclopedia
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which a carbon–carbon covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
is formed between the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
in presence of an electron donor in a free radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
process . The reaction product is a vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
. The reaction is named after the product of this reaction with acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, pinacol
Pinacol
Pinacol is a white solid organic compound.-Preparation:It may be produced by the pinacol coupling reaction from acetone:-Reactions:As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g...
(also 2,3-dimethyl-2,3-butanediol or tetramethylethylene glycol). The reaction is usually a homocoupling but intramolecular cross-coupling reactions are also possible. Pinacol was discovered by Wilhelm Rudolph Fittig
Wilhelm Rudolph Fittig
Wilhelm Rudolph Fittig was a German chemist. Fittig discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. He studied the action of sodium on ketones and hydrocarbons...
in 1859.
Reaction mechanism
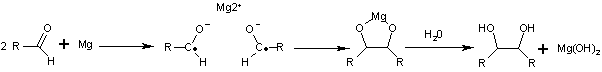
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is one-electron reduction
One-electron reduction
A one-electron reduction in organic chemistry involves the transfer of an electron from a metal to an organic substrate. It serves to differentiate between true organic reductions and other reductions such as hydride transfer reactions that actually involve two-electron species.The first...
of the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group by a reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
such as magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
to a ketyl
Ketyl
A ketyl group in organic chemistry is an anion radical with the general structure C-O. in which an oxygen radical is bonded directly to carbon. This radical is very unstable and appears in chemical reactions as a reactive intermediate...
radical anion species. Two ketyl groups react in a coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
yielding a vicinal diol with both hydroxyl groups deprotonated. Addition of water or another proton donor gives the diol. With magnesium as an electron donor, the initial reaction product is a 5-membered cyclic compound with the two oxygen atoms coordinated to the oxidized Mg2+ ion. This complex is also broken up by addition of water with formation of magnesium hydroxide
Magnesium hydroxide
Magnesium hydroxide is an inorganic compound with the chemical formula Mg2. As a suspension in water, it is often called milk of magnesia because of its milk-like appearance. The solid mineral form of magnesium hydroxide is known as brucite....
. The pinacol coupling can be followed up by a pinacol rearrangement
Pinacol rearrangement
The pinacol rearrangement or pinacol-pinacolone rearrangement is a method for converting a 1,2-diol to a carbonyl compound in organic chemistry. This 1,2-rearrangement takes place under acidic conditions...
. A related reaction is the McMurry reaction
McMurry reaction
The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to an alkene using titanium chloride compound such as titanium chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the...
, which uses titanium trichloride or titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
in conjunction with a reducing agent for the formation of the metal-diol complex, and which takes place with an additional deoxygenation reaction step in order to provide an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
product.
Scope
The Pinacol coupling of benzaldehydeBenzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
can be carried out in water with vanadium trichloride and aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
as co-reductant in order to maintain the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
. This heterogeneous reaction in water at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
yields 72% after 3 days with 56:44 dl
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
:meso
Meso compound
A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters it is not chiral. A meso compound is superimposable on its mirror image, and it does not produce a ""...
composition. In another system with benzaldehyde, Montmorillonite K-10
Montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that typically form in microscopic crystals, forming a clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite family, is a 2:1 clay, meaning that it has 2 tetrahedral sheets sandwiching a central...
and zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
in aqueous THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
under ultrasound
Ultrasound
Ultrasound is cyclic sound pressure with a frequency greater than the upper limit of human hearing. Ultrasound is thus not separated from "normal" sound based on differences in physical properties, only the fact that humans cannot hear it. Although this limit varies from person to person, it is...
the reaction time is reduced to 3 hours (composition 55:45) . On the other hand certain tartaric acid
Tartaric acid
Tartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
derivatives can be obtained with high diastereoselectivity in a system of samarium(II) iodide
Samarium(II) iodide
Samarium iodide is a green solid composed of samarium and iodine, with a melting point of 520 °C where the samarium atom has a coordination number of seven in a capped octahedral configuration...
and HMPA
Hexamethylphosphoramide
Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide having the formula [2N]3PO. This colorless liquid is a useful polar aprotic solvent and additive in organic synthesis.-Structure and reactivity:...
.
Two examples of pinacol coupling used in total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
are the Mukaiyama Taxol total synthesis
Mukaiyama Taxol total synthesis
The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis....
and the Nicolaou Taxol total synthesis
Nicolaou Taxol total synthesis
The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. This organic synthesis was included in Nicolaou's book, 'Classics in Total Synthesis'....
.
External links
- Conversion of benzophenone to benzopinacol in sunlight, Organic SynthesesOrganic SynthesesOrganic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
, Coll. Vol. 2, p.71 (1943); Vol. 14, p.8 (1934). - Conversion of acetone to pinacol hydrate with magnesium and mercury(II) chloride, Organic SynthesesOrganic SynthesesOrganic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
, Coll. Vol. 1, p.459 (1941); Vol. 5, p.87 (1925). - Pinacol MSDS

