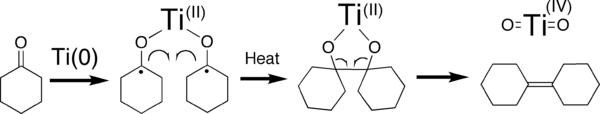
McMurry reaction
Encyclopedia
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which two ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
groups are coupled to an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
using titanium chloride
Titanium chloride
Titanium chloride may refer to:* Titanium tetrachloride , TiCl4* Titanium trichloride , TiCl3* Titanium dichloride , TiCl2...
compound such as titanium(III) chloride
Titanium(III) chloride
Titanium chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known...
and a reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
. The reaction is named after its co-discoverer, John E. McMurry
John E. McMurry
John McMurry, born July 27, 1942, in New York City, is Professor Emeritus in the Department of Chemistry and Chemical Biology at Cornell University. He received his Ph.D. working with Gilbert Stork...
. The McMurry reaction originally involved the use of a mixture TiCl3 and LiAlH4, which produces the active reagent(s). Related species have been developed involving the combination of TiCl3 or TiCl4 with various other reducing agents, including potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, and magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
. This reaction is related to the pinacol coupling reaction Pinacol coupling reaction
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
which also procedes by reductive coupling of carbonyl compounds.
Reaction mechanism
This reductive coupling can be viewed as involving two steps. First is the formation of a pinacolPinacol
Pinacol is a white solid organic compound.-Preparation:It may be produced by the pinacol coupling reaction from acetone:-Reactions:As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g...
ate (1,2-diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
ate) complex, a step which is equivalent to the pinacol coupling reaction
Pinacol coupling reaction
A pinacol coupling reaction is an organic reaction in which a carbon–carbon covalent bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process . The reaction product is a vicinal diol...
. The second step is the deoxygenation
Deoxygenation
Deoxygenation is a chemical reaction involving the removal of molecular oxygen from a reaction mixture or solvent, or the removal of oxygen atoms from a molecule.Classic representatives of deoxygenation are:...
of the pinacolate which yields the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. The second step exploits the oxophilicity
Oxophilicity
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of oxygen, often from organic compounds. The term is usually used to describe metal centers, commonly the early transition metals such as titanium, niobium, and tungsten. Oxophilic metals are...
of titanium.
Several mechanisms have been discussed for this reaction. Low-valent titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
species induce coupling of the carbonyls by single electron transfer
One-electron reduction
A one-electron reduction in organic chemistry involves the transfer of an electron from a metal to an organic substrate. It serves to differentiate between true organic reductions and other reductions such as hydride transfer reactions that actually involve two-electron species.The first...
to the carbonyl groups. The required low-valent titanium species are generated via reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, usually with zinc powder. This reaction is often performed in THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
because it solubilizes intermediate complexes, facilitates the electron transfer steps, and is not reduced under the reaction conditions. The nature of low-valent titanium species formed is varied as the products formed by reduction of the precursor titanium halide complex will naturally depend upon both the solvent (most commonly THF or DME) and the reducing agent employed: typically, lithium aluminum hydride, zinc-copper couple, zinc dust, magnesium-mercury amalgam, magnesium, or alkali metals. Bogdanovic and Bolte identified the nature and mode of action
Mechanism of action
In pharmacology, the term mechanism of action refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect...
of the active species in some classical McMurry systems. Unfortunately, such a titanium species is not clearly depicted in the "cartoon" shown above. In fact, the intermediacy of Ti(0) particles has been precluded by Bogdanovic and Bolte for some McMurry systems. It would benefit this page if that overtly incorrect cartoon were removed and replaced with any one of the many schemes in the references 4-6. The formation of titanium dioxide product is also incorrect in the "cartoon". Although it is true that titanium dioxide is usually the eventual fate of titanium used in these reactions, it is generally formed upon the aqueous workup of the reaction mixture.
Background and scope
The original publication by Mukaiyama demonstrated reductive coupling of ketones using reduced titanium reagents. McMurry and Fleming coupled retinalRetinal
Retinal, also called retinaldehyde or vitamin A aldehyde, is one of the many forms of vitamin A . Retinal is a polyene chromophore, and bound to proteins called opsins, is the chemical basis of animal vision...
to give carotene
Carotene
The term carotene is used for several related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but cannot be made by animals. Carotene is an orange photosynthetic pigment important for photosynthesis. Carotenes are all coloured to the human eye...
using a mixture of titanium trichloride and lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
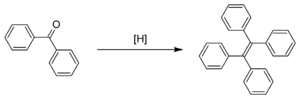
. Other symmetrical alkenes were prepared similarly, e.g. civetone
Civetone
Civetone is a cyclic ketone and one of the oldest perfume ingredients known. It is a pheromone sourced from the African Civet. It has a strong musky odor that becomes pleasant at extreme dilutions. Civetone is closely related to muscone, the principal odiferous compound found in musk, because both...
from adamantanone and tetraphenylethylene from benzophenone
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
. A McMurry reaction using titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
and zinc is employed in the synthesis of a first-generation molecular motor
Synthetic molecular motors
Synthetic molecular motors are molecular machines capable of rotation under energy input. Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion , some groups also use the term when referring to non-biological, non-peptide synthetic...
.
In another example, the Nicolaou's total synthesis of Taxol
Nicolaou Taxol total synthesis
The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. This organic synthesis was included in Nicolaou's book, 'Classics in Total Synthesis'....
uses this reaction, although coupling stops with the formation of a cis-diol, rather than an olefin. Optimized procedures employ the dimethoxyethane
Dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a clear, colorless, aprotic, and liquid ether that is used as a solvent. Dimethoxyethane is miscible with water.Dimethoxyethane is often used as a higher boiling...
complex of TiCl3 in combination with the Zn(Cu)
Zinc-copper couple
Zinc-copper couple is an alloy of zinc and copper that is employed as a reagent in organic synthesis. The “couple” was popularized after the report by Simmons and Smith in 1959 of its application as an activated source of zinc required for formation of an organozinc reagent in the Simmons-Smith...
.