
Oxocarbon
Encyclopedia
An oxocarbon or oxide of carbon is an inorganic compound
consisting only of carbon
and oxygen
.
The simplest and most common oxocarbons are carbon monoxide
(CO) and carbon dioxide
(CO2). Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide
(C3O2 or O=C=C=C=O) and mellitic anhydride
(C12O9).
While textbooks will often list only the first three, and rarely the fourth, a large number of other oxides are known today, most of them synthesized since the 1960s. Some of these new oxides are stable at room temperature. Some are metastable or stable only at very low temperatures, but decompose to simpler oxocarbons when warmed. Many are inherently unstable and can be observed only momentarily as intermediates in chemical reactions or are so reactive that they can exist only in the gas phase or under matrix isolation
conditions.
The inventory of oxocarbons appears to be steadily growing. The existence of graphene oxide
and of other stable polymer
ic carbon oxides with unbounded molecular structures suggests that many more remain to be discovered.
of carbon-containing substances and fermentation
of foods such as beer
and bread
. It was gradually recognized as a chemical substance, formerly called spiritus sylvestre ("forest spirit") or "fixed air", by various chemists in the 17th and 18th centuries.
Carbon monoxide may occur in combustion, too, and was used (though not recognized) since antiquity for the smelting
of iron
from its ore
s. Like the dioxide, it was described and studied in the West by various alchemist
s and chemists since the Middle Ages. Its true composition was discovered by William Cruikshank
in 1800.
Carbon suboxide was discovered by Brodie in 1873, by passing electric current through carbon dioxide.
The fourth "classical" oxide, mellitic anhydride
(C12O9), was apparently obtained by Liebig and Wöhler
in 1830 in their study of mellite ("honeystone")
, but was characterized only in 1913, by Meyer and Steiner.
Brodie also discovered in 1859 a sixth compound called graphite oxide, consisting of carbon and oxygen in ratios varying between 2:1 and 3:1; but the nature and molecular structure of this substance remained unknown until a few years ago, when it was renamed graphene oxide
and became a topic of research in nanotechnology
.
Notable examples of unstable or metastable oxides that were detected only in extreme situations are dicarbon monoxide
radical (:C=C=O), carbon trioxide
(CO3),, carbon tetroxide
, and 1,2-dioxetanedione
(C2O4). Some of these reactive carbon oxides were detected within molecular clouds in the interstellar medium
by rotational spectroscopy
.
Many hypothetical oxocarbons have been studied by theoretical methods but have yet to be detected. Examples include oxalic anhydride
(C2O3 or O=(C2O)=O), ethylene dione
(C2O2 or O=C=C=O) and other linear or cyclic polymers of carbon monoxide (-CO-)n (polyketone
s), and linear or cyclic polymers of carbon dioxide (-CO2-)n, such as the dimer 1,3-dioxetanedione
(C2O4) and the trimer 1,3,5-trioxanetrione
(C3O6).
while oxygen is divalent
, and in most oxocarbons (as in most other carbon compounds) each carbon atom may be bound
to four other atoms, while oxygen may be bound to at most two. Moreover, while carbon can connect to other carbons to form arbitrarily large chains or networks, chains of three or more oxygens are rarely if ever observed. Thus the known electrically neutral oxocarbons generally consist of one or more carbon skeletons (including cyclic
and aromatic structures) connected and terminated by oxide (-O-, =O) or peroxide (-O-O-) groups.
Carbon atoms with unsatisfied bonds are found in some oxides, such as the diradical C2O or :C=C=O; but these compounds are generally too reactive to be isolated in bulk. Loss or gain of electrons can result in monovalent negative oxygen (-), trivalent positive oxygen (≡), or trivalent negative carbon (≡). The last two are found in carbon monoxide, −C≡O+. Negative oxygen occurs in most oxocarbon anion
s.
Some higher member of this family have been detected in trace amounts in low-pressure gas phase and/or cryogenic matrix experiments, specifically for n = 7 and n = 17, 19, and 21.
When n is even, the molecules are believed to be in the triplet
(cumulene
-like) state, with the atoms connected by double bonds and an unfilled orbital in the first carbon — as in :C=C=O, :C=C=C=C=O, and, in general, :(C=)n=O. When n is odd, the triplet structure is believed to resonate
with a singlet (acetylene
-type) polar state with a negative charge on the carbon end and a positive one on the oxygen end, as in −C≡C-C≡O+, −C≡C-C≡C-C≡O+, and, in general, −(C≡C-)n/2C≡O+. Carbon monoxide itself follows this pattern: its predominant form is believed to be −C≡O+.
-type oxocarbons CnOn or (CO)n. They can be regarded as cyclic polymers of carbon monoxide, or n-fold ketones of n-carbon cycloalkanes. Carbon monoxide itself (CO) can be regarded as the first member. Theoretical studies indicate that ethylene dione (C2O2 or O=C=C=O) and cyclopropanetrione C3O3 do not exist.. The next three members — C4O4
, C5O5
, and C6O6
— are theoretically possible, but are expected to be quite unstable, and so far they have been synthesized only in trace amounts.
On the other hand, the anions of these oxocarbons are quite stable, and some of them have been known since the 19th century. They are
The cyclic oxide C6O6 also forms the stable anions of tetrahydroxybenzoquinone
(C6O64−) and hexahydroxybenzene
(C6O66−), The aromaticity
of these anions has been studied using theoretical methods.
Many relatives of these oxides have been investigated theoretically, and some are expected to be stable, such as other carbonate and oxalate esters of tetrahydroxy-1,2-benzoquinone and of the rhodizonic, croconic, squaric, and deltic acids.
, with 3:2 carbon:oxygen atomic ratio. The polymer is believed to be a linear chain of fused six-membered lactone
rings, with a continuous carbon backbone of alternating single and double bonds. Physical measurements indicate that the mean number of units per molecule is about 5–6, depending on the formation temperature.
Carbon monoxide compressed to 5 GPa
in a diamond anvil cell
yields a somewhat similar reddish polymer with a slightly higher oxygen content, which is metastable at room conditions. It is believed that CO disproportionates
in the cell to a mixture of CO2 and C3O2; the latter forms a polymer similar to the one described above (but with a more irregular structure), that traps some of the CO2 in its matrix..
Another carbon-oxygen polymer, with C:O ratio 5:1 or higher, is the classical graphite oxide and its single-sheet version graphene oxide
.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
consisting only of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
.
The simplest and most common oxocarbons are carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(CO) and carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2). Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide
Carbon suboxide
Carbon suboxide, or tricarbon dioxide, is an oxide of carbon with chemical formula C3O2 or O=C=C=C=O. Its four cumulative double bonds make it a cumulene...
(C3O2 or O=C=C=C=O) and mellitic anhydride
Mellitic anhydride
Mellitic anhydride, anhydride of mellitic acid, is organic compound with formula C12O9.Mellitic anhydride is oxide of carbon , like CO2, CO, and C3O2. It is white sublimable solid, apparently obtained by Liebig and Wöhler in 1830 in their study of mellite , who assigned it the formula C4O3. The...
(C12O9).
 |  |  | |||||
| CO Carbon monoxide | CO2 Carbon dioxide | C3O2 Carbon suboxide | C12O9 Mellitic anhydride |
While textbooks will often list only the first three, and rarely the fourth, a large number of other oxides are known today, most of them synthesized since the 1960s. Some of these new oxides are stable at room temperature. Some are metastable or stable only at very low temperatures, but decompose to simpler oxocarbons when warmed. Many are inherently unstable and can be observed only momentarily as intermediates in chemical reactions or are so reactive that they can exist only in the gas phase or under matrix isolation
Matrix Isolation
Matrix isolation is an experimental technique used in chemistry and physics which generally involves a material being trapped within an unreactive matrix. A host matrix is a continuous solid phase in which guest particles are embedded. The guest is said to be isolated within the host matrix...
conditions.
The inventory of oxocarbons appears to be steadily growing. The existence of graphene oxide
Graphene oxide
Graphite oxide, formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers...
and of other stable polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
ic carbon oxides with unbounded molecular structures suggests that many more remain to be discovered.
Overview
Carbon dioxide (CO2) occurs widely in nature, and was incidentally produced by humans since pre-historical times, by the combustionCombustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of carbon-containing substances and fermentation
Fermentation (food)
Fermentation in food processing typically is the conversion of carbohydrates to alcohols and carbon dioxide or organic acids using yeasts, bacteria, or a combination thereof, under anaerobic conditions. Fermentation in simple terms is the chemical conversion of sugars into ethanol...
of foods such as beer
Beer
Beer is the world's most widely consumed andprobably oldest alcoholic beverage; it is the third most popular drink overall, after water and tea. It is produced by the brewing and fermentation of sugars, mainly derived from malted cereal grains, most commonly malted barley and malted wheat...
and bread
Bread
Bread is a staple food prepared by cooking a dough of flour and water and often additional ingredients. Doughs are usually baked, but in some cuisines breads are steamed , fried , or baked on an unoiled frying pan . It may be leavened or unleavened...
. It was gradually recognized as a chemical substance, formerly called spiritus sylvestre ("forest spirit") or "fixed air", by various chemists in the 17th and 18th centuries.
Carbon monoxide may occur in combustion, too, and was used (though not recognized) since antiquity for the smelting
Smelting
Smelting is a form of extractive metallurgy; its main use is to produce a metal from its ore. This includes iron extraction from iron ore, and copper extraction and other base metals from their ores...
of iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
from its ore
Ore
An ore is a type of rock that contains minerals with important elements including metals. The ores are extracted through mining; these are then refined to extract the valuable element....
s. Like the dioxide, it was described and studied in the West by various alchemist
Alchemist
An alchemist is a person who practices alchemy. Alchemist may also refer to:-People and groups:*The Alchemist , a hip hop music producer and rapper*Alchemist , an Australian progressive metal band...
s and chemists since the Middle Ages. Its true composition was discovered by William Cruikshank
William Cruickshank (chemist)
William Cruickshank was a Scottish military surgeon and chemist, and professor of chemistry at the Royal Military Academy, Woolwich.-Career:...
in 1800.
Carbon suboxide was discovered by Brodie in 1873, by passing electric current through carbon dioxide.
The fourth "classical" oxide, mellitic anhydride
Mellitic anhydride
Mellitic anhydride, anhydride of mellitic acid, is organic compound with formula C12O9.Mellitic anhydride is oxide of carbon , like CO2, CO, and C3O2. It is white sublimable solid, apparently obtained by Liebig and Wöhler in 1830 in their study of mellite , who assigned it the formula C4O3. The...
(C12O9), was apparently obtained by Liebig and Wöhler
Friedrich Wöhler
Friedrich Wöhler was a German chemist, best known for his synthesis of urea, but also the first to isolate several chemical elements.-Biography:He was born in Eschersheim, which belonged to aau...
in 1830 in their study of mellite ("honeystone")
Mellite
Mellite, also called honeystone, is an unusual mineral being also an organic chemical. Chemically identified as an aluminium salt of mellitic acid; that is, aluminium benzene hexacarboxylate hydrate, with the chemical formula Al2C66·16H2O....
, but was characterized only in 1913, by Meyer and Steiner.
Brodie also discovered in 1859 a sixth compound called graphite oxide, consisting of carbon and oxygen in ratios varying between 2:1 and 3:1; but the nature and molecular structure of this substance remained unknown until a few years ago, when it was renamed graphene oxide
Graphene oxide
Graphite oxide, formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers...
and became a topic of research in nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
.
Notable examples of unstable or metastable oxides that were detected only in extreme situations are dicarbon monoxide
Dicarbon monoxide
Dicarbon monoxide is an extremely reactive molecule that contains two carbon atoms and one oxygen atom. Dicarbon monoxide, covalently bonded, is a product of the photolysis of carbon suboxide. It is closely related to CO, CO2 and C3O2, and other oxocarbons.It is stable enough to observe reactions...
radical (:C=C=O), carbon trioxide
Carbon trioxide
Carbon trioxide is an unstable oxide of carbon . Three possible isomers of carbon trioxide, denoted Cs, D3h, and C2v, have been most studied by theoretical methods, and the C2v state has been shown to be the ground state of the molecule.Carbon trioxide should not be confused with the stable...
(CO3),, carbon tetroxide
Carbon tetroxide
Carbon tetroxide is a highly unstable oxide of carbon with formula . It was proposed as an intermediate in the O-atom exchange between carbon dioxide and oxygen at high temperatures....
, and 1,2-dioxetanedione
1,2-Dioxetanedione
The chemical compound 1,2-dioxetanedione, or 1,2-dioxacyclobutane-3,4-dione, often called peroxyacid ester, is an unstable oxide of carbon with formula C2O4...
(C2O4). Some of these reactive carbon oxides were detected within molecular clouds in the interstellar medium
Interstellar medium
In astronomy, the interstellar medium is the matter that exists in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, dust, and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space...
by rotational spectroscopy
Rotational spectroscopy
Rotational spectroscopy or microwave spectroscopy studies the absorption and emission of electromagnetic radiation by molecules associated with a corresponding change in the rotational quantum number of the molecule...
.
Many hypothetical oxocarbons have been studied by theoretical methods but have yet to be detected. Examples include oxalic anhydride
Oxalic anhydride
Oxalic anhydride or ethanedioic anhydride, also called oxiranedione, is a hypothetical organic compound with the formula C2O3, which can be viewed as the anhydride of oxalic acid or the two-fold ketone of ethylene oxide. It is an oxide of carbon .The simple compound apparently has yet to be...
(C2O3 or O=(C2O)=O), ethylene dione
Ethylene dione
Ethylene dione or ethylenedione, also called dicarbon dioxide, ethenedione, or ethene 1,2-dione, is the name given to a hypothetical chemical compound with the formula C2O2 or O=C=C=O. It would be an oxide of carbon , specifically a dimer of carbon monoxide...
(C2O2 or O=C=C=O) and other linear or cyclic polymers of carbon monoxide (-CO-)n (polyketone
Polyketone
Polyketone Density1240 kg/m3Young's modulus 1500 MPaTensile strength 55 MPaElongation @ break350 %notch test20 kJ/m2Glass temperature15°Cmelting point220°CVicat B205heat transfer coefficient 0.27 W/...
s), and linear or cyclic polymers of carbon dioxide (-CO2-)n, such as the dimer 1,3-dioxetanedione
1,3-Dioxetanedione
The chemical compound 1,3-dioxetanedione, or 1,3-dioxacyclobutane-2,4-dione is a hypothetical oxide of carbon with formula C2O4. It can be considered a cyclic dimer of carbon dioxide or as a double ketone of 1,3-dioxetane ....
(C2O4) and the trimer 1,3,5-trioxanetrione
1,3,5-Trioxanetrione
The chemical compound 1,3,5-trioxanetrione, or 1,3,5-trioxacyclohexane-2,4,6-trione is a hypothetical oxide of carbon with formula C3O6. It can be considered a cyclic trimer of carbon dioxide or as a triple ketone of 1,3,5-trioxane .Theoretical calculations indicate that the compound is unstable...
(C3O6).
 |  |  |  |  | |||||
| C2O3 Oxalic anhydride | C2O4 1,2-Dioxetane- dione | C2O4 1,3-Dioxetane- dione | C3O6 1,3,5-Trioxane- trione | C2O2 Ethylene dione |
General structure
Normally carbon is tetravalentValence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
while oxygen is divalent
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
, and in most oxocarbons (as in most other carbon compounds) each carbon atom may be bound
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
to four other atoms, while oxygen may be bound to at most two. Moreover, while carbon can connect to other carbons to form arbitrarily large chains or networks, chains of three or more oxygens are rarely if ever observed. Thus the known electrically neutral oxocarbons generally consist of one or more carbon skeletons (including cyclic
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
and aromatic structures) connected and terminated by oxide (-O-, =O) or peroxide (-O-O-) groups.
Carbon atoms with unsatisfied bonds are found in some oxides, such as the diradical C2O or :C=C=O; but these compounds are generally too reactive to be isolated in bulk. Loss or gain of electrons can result in monovalent negative oxygen (-), trivalent positive oxygen (≡), or trivalent negative carbon (≡). The last two are found in carbon monoxide, −C≡O+. Negative oxygen occurs in most oxocarbon anion
Oxocarbon anion
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula CxOyn− for some integers x, y, and n....
s.
Linear carbon dioxides
One family of carbon oxides has the general formula CnO2, or O=(C=)nO — namely, a linear chain of carbon atoms, capped by oxygen atoms at both ends. The first members are- CO2 or O=C=O, the well-known carbon dioxide.
- C2O2 or O=C=C=O, the extremely unstable ethylene dioneEthylene dioneEthylene dione or ethylenedione, also called dicarbon dioxide, ethenedione, or ethene 1,2-dione, is the name given to a hypothetical chemical compound with the formula C2O2 or O=C=C=O. It would be an oxide of carbon , specifically a dimer of carbon monoxide...
. - C3O2 or O=C=C=C=O, the metastable carbon suboxideCarbon suboxideCarbon suboxide, or tricarbon dioxide, is an oxide of carbon with chemical formula C3O2 or O=C=C=C=O. Its four cumulative double bonds make it a cumulene...
or tricarbon dioxide. - C4O2 or O=C=C=C=C=O, tetracarbon dioxideTetracarbon dioxideTetracarbon dioxide is an oxide of carbon, a chemical compound of carbon and oxygen, with chemical formula C4O2 or O=C=C=C=C=O. It can be regarded as butatriene dione, the double ketone of butatriene — more precisely 1,2,3-butatriene-1,4-dione....
or 1,2,3-Butatriene-1,4-dione - C5O2 or O=C=C=C=C=C=O, pentacarbon dioxidePentacarbon dioxidePentacarbon dioxide, officially penta-1,2,3,4-tetraene-1,5-dione, is an oxide of carbon with formula C5O2 or O=C=C=C=C=C=O....
, stable in solution at room temp. and pure up to -90°C.
Some higher member of this family have been detected in trace amounts in low-pressure gas phase and/or cryogenic matrix experiments, specifically for n = 7 and n = 17, 19, and 21.
Linear carbon monoxides
Another family of oxocarbons are the linear carbon monoxides CnO. The first member, ordinary carbon monoxide CO, seems to be the only one that is stable in the pure state at room temperature. Photolysis of the linear carbon dioxides in a cryogenic matrix leads to loss of CO, resulting in detectable amounts of even-numbered monoxides such as C2O, C4O, and C6O. The members up to n=9 have also been obtained by electrical discharge on gasous C3O2 diluted in argon. The first three members have been detected in interstellar space.When n is even, the molecules are believed to be in the triplet
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
(cumulene
Cumulene
A cumulene is a chemical compound with two or more cumulative double bonds, for example butatriene , H2C=C=C=CH2. Unlike alkanes and most alkenes, cumulenes tend to be rigid, which makes them appealing for molecular nanotechnology. Polyynes are another kind of rigid carbon chains...
-like) state, with the atoms connected by double bonds and an unfilled orbital in the first carbon — as in :C=C=O, :C=C=C=C=O, and, in general, :(C=)n=O. When n is odd, the triplet structure is believed to resonate
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
with a singlet (acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
-type) polar state with a negative charge on the carbon end and a positive one on the oxygen end, as in −C≡C-C≡O+, −C≡C-C≡C-C≡O+, and, in general, −(C≡C-)n/2C≡O+. Carbon monoxide itself follows this pattern: its predominant form is believed to be −C≡O+.
Radialene-type cyclic polyketones
Another family of oxocarbons that has attracted special attention are the cyclic radialeneRadialene
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl group are also called radialenes...
-type oxocarbons CnOn or (CO)n. They can be regarded as cyclic polymers of carbon monoxide, or n-fold ketones of n-carbon cycloalkanes. Carbon monoxide itself (CO) can be regarded as the first member. Theoretical studies indicate that ethylene dione (C2O2 or O=C=C=O) and cyclopropanetrione C3O3 do not exist.. The next three members — C4O4
Cyclobutanetetrone
Cyclobutanetetrone, also called tetraoxocyclobutane, is a hypothetical organic compound with formula C4O4 or 4, the fourfold ketone of cyclobutane. It would be an oxide of carbon, indeed a tetramer of carbon monoxide....
, C5O5
Cyclopentanepentone
Cyclopentanepentone, also known as leuconic acid, is a hypothetical organic compound with formula C5O5, the fivefold ketone of cyclopentane...
, and C6O6
Cyclohexanehexone
Cyclohexanehexone, also known as hexaketocyclohexane and triquinoyl, is a organic compound with formula C6O6, the sixfold ketone of cyclohexane...
— are theoretically possible, but are expected to be quite unstable, and so far they have been synthesized only in trace amounts.
 | 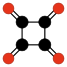 | 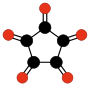 | 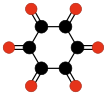 | ||||
| (CO)3 Cyclopropane- trione | (CO)4 Cyclobutane- tetrone | (CO)5 Cyclopentane- pentone | (CO)6 Cyclohexane hexone |
On the other hand, the anions of these oxocarbons are quite stable, and some of them have been known since the 19th century. They are
- C2O22−, acetylenediolAcetylenediolAcetylenediol, or ethynediol, is a chemical substance with formula HO-C≡C-OH. It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal H2H is well known.-Detection:...
ate (Weiss and Büchner, 1963), - C3O32−, deltateDeltic acidDeltic acid or dihydroxycyclopropenone is a chemical substance with the chemical formula C3O2. It can be viewed as a ketone and double alcohol of cyclopropene...
(Eggerding and West, 1976), - C4O42−, squarateSquaric acidSquaric acid, also called quadratic acid, because its four carbon atoms approximately form a square, is an organic compound with chemical formula 424....
(Cohen and others, 1959), - C5O52−, croconateCroconic acidCroconic acid or 4,5-dihydroxycyclopentenetrione is a chemical compound with formula C5H2O5 or 32. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms...
(Gmelin, 1825), and - C6O62−, rhodizonateRhodizonic acidRhodizonic acid is a chemical compound with formula C6H2O6 or 42. It can be seen as a two-fold alcohol and four-fold ketone of cyclohexene, more precisely 5,6-dihydroxycyclohex-5-ene-1,2,3,4-tetrone....
(Heller, 1837).
The cyclic oxide C6O6 also forms the stable anions of tetrahydroxybenzoquinone
Tetrahydroxy-1,4-benzoquinone
Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydoxybenzoquinone, or tetrahydroxyquinone , is an organic compound with formula C6O24...
(C6O64−) and hexahydroxybenzene
Benzenehexol
Benzenehexol, also called hexahydroxybenzene, is an organic compound with formula C6H6O6 or C66. It is a six-fold alcohol of benzene. The product is also erroneously called hexaphenol, but this name has been used also for other substances....
(C6O66−), The aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
of these anions has been studied using theoretical methods.
New oxides
Many new stable or metastable oxides have been synthesized since the 1960s, such as:- C10O8, benzoquinonetetracarboxylic dianhydrideBenzoquinonetetracarboxylic dianhydrideBenzoquinonetetracarboxylic dianhydride is an organic compound with formula which can be seen as the result of removing two molecules of water from benzoquinonetetracarboxylic acid....
(Hammond, 1963). - C6O6, ethylenetetracarboxylic dianhydrideEthylenetetracarboxylic dianhydrideEthylenetetracarboxylic dianhydride is a chemical compound with formula , that can be seen as the twofold anhydride of ethylenetetracarboxylic acid . Its molecular backbone consists of two five-atom maleic anhydride rings, each containing one oxygen atom and four carbon atoms, sharing a pair of...
, a stable isomer of cyclohexanehexone (Sauer and others, 1967). - C12O12 or C6(C2O4)3, hexahydroxybenzene trisoxalateHexahydroxybenzene trisoxalateHexahydroxybenzene trisoxalate is a chemical compound, an oxide of carbon with formula . Its molecule consists of a benzene core with the six hydrogen atoms replaced by three oxalate groups. It can be seen as a sixfold ester of benzenehexol and oxalic acid....
(Verter and Dominic, 1967); stable as a tetrahydrofuranTetrahydrofuranTetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
solvate. - C10O10 or C6O2(C2O4)2, tetrahydroxy-1,4-benzoquinone bisoxalateTetrahydroxy-1,4-benzoquinone bisoxalateTetrahydroxy-1,4-benzoquinone bisoxalate is a chemical compound, an oxide of carbon with formula . Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two oxalate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and oxalic acid.The...
(Verter and others, 1968); stable as a tetrahydrofuran solvate. - C8O8 or C6O2(CO3)2, tetrahydroxy-1,4-benzoquinone biscarbonateTetrahydroxy-1,4-benzoquinone biscarbonateTetrahydroxy-1,4-benzoquinone biscarbonate is a chemical compound, an oxide of carbon with formula . Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two carbonate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and carbonic...
(Nallaiah, 1984); decomposes at about 45–53°C. - C9O9 or C6(CO3)3, hexahydroxybenzene triscarbonateHexahydroxybenzene triscarbonateHexahydroxybenzene triscarbonate is a chemical compound, an oxide of carbon with formula . Its molecule consists of a benzene core with the six hydrogen atoms replaced by three carbonate groups. It can be seen as a sixfold ester of hexahydroxybenzene and carbonic acid.The compound was obtained by C...
(Nallaiah, 1984); decomposes at about 45–53°C. - C24O6, a cyclic trimer of the biradicalRadical (chemistry)Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
3,4-dialkynyl-3-cyclobutene1,2-dione -C≡C-(C4O2)-C≡C- (Rubin and others, 1990); - C32O8, a tetramer of 3,4-dialkynyl-3-cyclobutene1,2-dione (Rubin and others, 1990);
- C4O6, dioxane tetraketoneDioxane tetraketoneDioxane tetraketone is an organic compound with the formula C4O6. It is an oxide of carbon , which can be viewed as the fourfold ketone of dioxane...
or dimeric oxalic anhydride (Strazzolini and others, 1998); stable in Et2ODiethyl etherDiethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
at −30°C, decomposes at 0°C. - C12O6, hexaoxotricyclobutabenzeneHexaoxotricyclobutabenzeneHexaoxotricyclobutabenzene is an organic compound with formula C12O6. It can be viewed as the sixfold ketone of tricyclobutabenzene. It is one of the novel oxides of carbon, detected by 13C NMR by T. Hamura and others in 2006....
(Hamura and others, 2006)
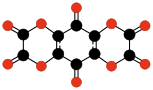 | |||||
| C10O8 Benzoquinone- tetracarboxylic dianhydride | C6O6 Ethylene- tetracarboxylic dianhydride | C10O10 Tetrahydroxy- 1,4-benzoquinone bisoxalate | |||
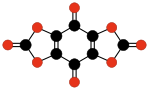 |  | 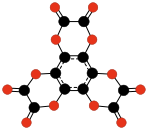 | |||
| C8O8 Tetrahydroxy- 1,4-benzoquinone biscarbonate | C4O6 Dioxane tetraketone | C12O12 Hexahydroxybenzene trisoxalate | |||
 |  |  | |||
| C9O9 Hexahydroxybenzene triscarbonate | C24O6 Tris(3,4-dialkynyl- 3-cyclobutene- 1,2-dione) | C32O8 Tetrakis(3,4-dialkynyl- 3-cyclobutene- 1,2-dione) | |||
 | |||||
| C12O6 Hexaoxotricyclo- butabenzene |
Many relatives of these oxides have been investigated theoretically, and some are expected to be stable, such as other carbonate and oxalate esters of tetrahydroxy-1,2-benzoquinone and of the rhodizonic, croconic, squaric, and deltic acids.
Polymeric carbon oxides
Carbon suboxide spontaneously polymerizes at room temperature into a carbon-oxygen polymerPolymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
, with 3:2 carbon:oxygen atomic ratio. The polymer is believed to be a linear chain of fused six-membered lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
rings, with a continuous carbon backbone of alternating single and double bonds. Physical measurements indicate that the mean number of units per molecule is about 5–6, depending on the formation temperature.
 | 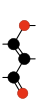 |  |  | ||||||
| Terminating and repeating units of polymeric C3O2. | |||||||||
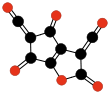 | 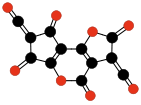 |  | 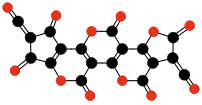 | ||||||
| Oligomers of C3O2 with 3 to 6 units. | |||||||||
Carbon monoxide compressed to 5 GPa
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
in a diamond anvil cell
Diamond anvil cell
A diamond anvil cell is a device used in scientific experiments. It allows compressing a small piece of material to extreme pressures, which can exceed 3,000,000 atmospheres ....
yields a somewhat similar reddish polymer with a slightly higher oxygen content, which is metastable at room conditions. It is believed that CO disproportionates
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
in the cell to a mixture of CO2 and C3O2; the latter forms a polymer similar to the one described above (but with a more irregular structure), that traps some of the CO2 in its matrix..
Another carbon-oxygen polymer, with C:O ratio 5:1 or higher, is the classical graphite oxide and its single-sheet version graphene oxide
Graphene oxide
Graphite oxide, formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers...
.

