Matrix Isolation
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
which generally involves a material being trapped within an unreactive matrix. A host matrix is a continuous solid phase in which guest particles (atoms, molecules, ions, etc.) are embedded. The guest is said to be isolated within the host matrix. Initially the term matrix-isolation was used to describe the placing of a chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
in any unreactive material, often polymers or resins, but more recently has referred specifically to gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es in low-temperature solids. A typical matrix isolation experiment involves a guest sample being diluted in the gas phase with the host material, usually a noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
or nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. This mixture is then deposited on a cold window, often cooled to 10 kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
s or below. The sample may then be studied using various spectroscopic
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
procedures.
Experiments
The transparent window, on to which the sample is deposited, is usually cooled using a compressed heliumHelium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
or similar refrigerant. Experiments must be performed under a high vacuum to prevent contaminates from unwanted gases freezing to the cold window. Lower temperatures are preferred, due to the improved rigidity and “glassiness” of the matrix material. Noble gases such as argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
are used not just because of their unreactivity but also because of their broad optical transparency in the solid state. Mono-atomic gases have relatively simple face-centered cubic (fcc)
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
, which can make interpretations of the site occupancy and crystal-field splitting
Crystal field theory
Crystal field theory is a model that describes the electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes,...
of the guest easier. In some cases a reactive
Reactive
Reactive may refer to:*Generally, capable of having a reaction*Reactance , the imaginary component of AC impedance*Reactive mind*Reactive programming...
material, for example, methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
or ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, may be used as the host material so that the reaction of the host with the guest species may be studied.
Using the matrix isolation technique, short-lived, highly-reactive species such as radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
ions and reaction intermediates may be observed and identified by spectroscopic means. For example, the solid noble gas krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
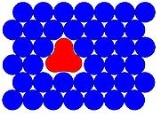
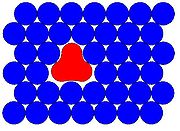
can be used to form an inert matrix within which a reactive F3- ion can sit in chemical isolation. A species may be created chemically before deposition, or after by photochemical
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
means. The technique may be used to simulate a species in the gas phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
without rotational and translational interference. The low temperatures also help to produce simpler spectra, since only the lower electronic and vibrational quantum states are populated.
History
Matrix isolation has its origins in the first half of the 20th century with the experiments by photo-chemists and physicists freezing samples in liquefied gases. The earliest isolation experiments involved the freezing of species in transparent, low temperature organic glassGlass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
es. The modern matrix isolation technique was developed extensively during the 1950s, in particular by George C. Pimentel
George C. Pimentel
George Claude Pimentel was the inventor of the chemical laser. He also developed the modern technique of matrix isolation in low-temperature chemistry. In theoretical chemistry, he proposed the three-centre four-electron bond which is now accepted as the best simple model of hypervalent...
. He initially used higher-boiling inert gases like xenon and nitrogen as the host material, and is often said to be the "father of matrix isolation".
See also
- Host-guest chemistryHost-guest chemistryIn supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
- Inert gases
- Van der Waals interactions
- RadicalsRadical (chemistry)Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...

