N-Bromosuccinimide
Encyclopedia
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution
and electrophilic addition
reactions
in organic chemistry
. NBS can be considered a convenient source of cationic bromine
.
. The NBS product precipitates out and can be collected by filtration.
Crude NBS gives better yield in the Wohl-Ziegler reaction
. In other cases, impure NBS (slightly yellow-colored) may give unreliable results. It can be purified by recrystallization from 90-95 °C water.
2. The preferred conditions are the portionwise addition of NBS to a solution of the alkene in 50% aqueous DMSO
, DME
, THF
, or tert-butanol
at 0°C. Formation of a bromonium ion and immediate attack by water gives strong Markovnikov addition
and anti stereochemical selectivities.
Side reactions include the formation of α-bromo-ketones and dibromo compounds. These can be minimized by the use of freshly recrystallize
d NBS.
With the addition of nucleophile
s, instead of water, various bifunctional alkanes can be synthesized.
CCl4
with a radical initiator—usually azo-bis-isobutyronitrile (AIBN) or benzoyl peroxide
—, irradiation, or both to effect radical
initiation
. The allylic and benzylic radical intermediates formed during this reaction are more stable than other carbon radicals and the major products are allylic and benzylic bromides. This is also called the Wohl-Ziegler reaction
.
The carbon tetrachloride
must be maintained anhydrous throughout the reaction, as the presence of water
may likely hydrolyze the desired product. Barium carbonate
is often added to maintain anhydrous and acid-free conditions.
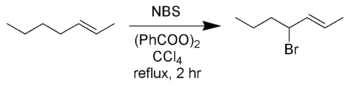
In the above reaction, while a mixture of isomeric allylic bromide products are possible, only one is created due to the greater stability of the 4-position radical over the methyl-centered radical.
The reaction of enolates, enol ether
s, or enol acetates with NBS is the preferred method of α-bromination as it is high-yielding with few side-products.
s, aniline
s, and various aromatic heterocycles, can be brominated using NBS. Using DMF
as the solvent gives high levels of para-selectivity.
, reacts with primary amide
s to produce a carbamate
via the Hofmann rearrangement
.
et al. found that one can selectively oxidize
secondary alcohol
s in the presence of primary alcohols using NBS in aqueous dimethoxyethane
(DME).
In general, reactions involving NBS are exothermic. Therefore, extra precautions should be taken when used on a large scale.
Radical substitution
In organic chemistry, a radical substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.The reaction always involves at least two steps, and possibly a third....
and electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
reactions
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. NBS can be considered a convenient source of cationic bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
.
Preparation
NBS is commercially available. It can also be synthesized in the laboratory. To do so, sodium hydroxide and bromine are added to an ice-water solution of succinimideSuccinimide
Succinimide is a cyclic imide with the formula C4H5NO2. It is used in a variety of organic syntheses, as well as in some industrial silver plating processes.-Succinimides:...
. The NBS product precipitates out and can be collected by filtration.
Crude NBS gives better yield in the Wohl-Ziegler reaction
Wohl-Ziegler reaction
The Wohl-Ziegler reactionis a chemical reaction that involves the allylic or benzylic bromination of hydrocarbons using an N-bromoimide and a radical initiator.Best yields are achieved with N-bromosuccinimide in carbon tetrachloride solvent...
. In other cases, impure NBS (slightly yellow-colored) may give unreliable results. It can be purified by recrystallization from 90-95 °C water.
Addition to alkenes
NBS will react with alkenes 1 in aqueous solvents to give bromohydrinsHalohydrin formation reaction
The halohydrin formation reaction is a chemical reaction in which a halogen is added to an alkene in aqueous solution to form a halohydrin. The reaction is a form of electrophilic addition; it is similar to the halogen addition reaction....
2. The preferred conditions are the portionwise addition of NBS to a solution of the alkene in 50% aqueous DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, DME
Dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a clear, colorless, aprotic, and liquid ether that is used as a solvent. Dimethoxyethane is miscible with water.Dimethoxyethane is often used as a higher boiling...
, THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
, or tert-butanol
Tert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
at 0°C. Formation of a bromonium ion and immediate attack by water gives strong Markovnikov addition
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
and anti stereochemical selectivities.
Side reactions include the formation of α-bromo-ketones and dibromo compounds. These can be minimized by the use of freshly recrystallize
Recrystallization (chemistry)
-Chemistry:In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted , which includes recrystallization...
d NBS.
With the addition of nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s, instead of water, various bifunctional alkanes can be synthesized.
Allylic and benzylic bromination
Standard conditions for using NBS in allylic and/or benzylic bromination involves refluxing a solution of NBS in anhydrousAnhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
CCl4
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
with a radical initiator—usually azo-bis-isobutyronitrile (AIBN) or benzoyl peroxide
Benzoyl peroxide
Benzoyl peroxide is an organic compound in the peroxide family. It consists of two benzoyl groups bridged by a peroxide link. Its structural formula is [C6H5C]2O2. It is one of the most important organic peroxides in terms of applications and the scale of its production...
—, irradiation, or both to effect radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
initiation
Initiation (chemistry)
In chemistry, initiation is a chemical reaction that triggers one or more secondary reactions. Often the initiation reaction generates a reactive intermediate from a stable molecule which is then involved in secondary reactions. In polymerisation, initiation is followed by a chain reaction and...
. The allylic and benzylic radical intermediates formed during this reaction are more stable than other carbon radicals and the major products are allylic and benzylic bromides. This is also called the Wohl-Ziegler reaction
Wohl-Ziegler reaction
The Wohl-Ziegler reactionis a chemical reaction that involves the allylic or benzylic bromination of hydrocarbons using an N-bromoimide and a radical initiator.Best yields are achieved with N-bromosuccinimide in carbon tetrachloride solvent...
.
The carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
must be maintained anhydrous throughout the reaction, as the presence of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
may likely hydrolyze the desired product. Barium carbonate
Barium carbonate
Barium carbonate , also known as witherite, is a chemical compound used in rat poison, bricks, ceramic glazes and cement.Witherite crystallizes in the orthorhombic system...
is often added to maintain anhydrous and acid-free conditions.
In the above reaction, while a mixture of isomeric allylic bromide products are possible, only one is created due to the greater stability of the 4-position radical over the methyl-centered radical.
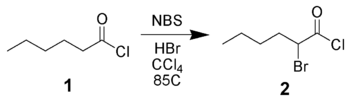
Bromination of carbonyl derivatives
NBS can α-brominate carbonyl derivatives via either a radical pathway (as above) or via acid-catalysis. For example, hexanoyl chloride 1 can be brominated in the alpha-position by NBS using acid catalysis.The reaction of enolates, enol ether
Enol ether
An enol ether is an alkene with an alkoxy substituent. The general structure is R_1R_2C=CR_3-O-R_4 with R an alkyl or an aryl group. Enol ethers and enamines are so-called activated alkenes or electron rich alkenes because the oxygen atom donates electrons to the double bond by forming a resonance...
s, or enol acetates with NBS is the preferred method of α-bromination as it is high-yielding with few side-products.
Bromination of aromatic derivatives
Electron-rich aromatic compounds, such as phenolPhenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s, aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s, and various aromatic heterocycles, can be brominated using NBS. Using DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
as the solvent gives high levels of para-selectivity.
Hofmann rearrangement
NBS, in the presence of a strong base, such as DBUDBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
, reacts with primary amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s to produce a carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
via the Hofmann rearrangement
Hofmann rearrangement
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom.The reaction is named after its discoverer: August Wilhelm von Hofmann...
.
Selective oxidation of alcohols
It is uncommon, but possible for NBS to oxidize alcohols. E. J. CoreyElias James Corey
Elias James Corey is an American organic chemist. In 1990 he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis...
et al. found that one can selectively oxidize
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
secondary alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s in the presence of primary alcohols using NBS in aqueous dimethoxyethane
Dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a clear, colorless, aprotic, and liquid ether that is used as a solvent. Dimethoxyethane is miscible with water.Dimethoxyethane is often used as a higher boiling...
(DME).
Oxidative decarboxylation of amino acids
NBS electrophilically brominates the amine, which is followed by decarboxylation and release of an imine. Hydrolysis of the imine yields an aldehyde and ammonia. (c.f. with non oxidative PLP dependant decarboxylation)Precautions
Although NBS is easier and safer to handle than bromine, precautions should be taken to avoid inhalation. NBS should be stored in a refrigerator. NBS will decompose over time giving off bromine. Pure NBS is white, but it is often found to be off-white or brown colored by bromine.In general, reactions involving NBS are exothermic. Therefore, extra precautions should be taken when used on a large scale.