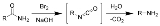
Hofmann rearrangement
Overview
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
reaction of a primary amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
to a primary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
with one fewer carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom.
The reaction is named after its discoverer: August Wilhelm von Hofmann
August Wilhelm von Hofmann
August Wilhelm von Hofmann was a German chemist.-Biography:Hofmann was born at Gießen, Grand Duchy of Hesse. Not intending originally to devote himself to physical science, he first took up the study of law and philology at Göttingen. But he then turned to chemistry, and studied under Justus von...
. This reaction is also sometimes called the Hofmann degradation, and should not be confused with the Hofmann elimination
Hofmann elimination
Hofmann elimination is a process where an amine is reacted to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat.After the first step, a quaternary ammonium iodide salt is created...
.
The reaction of bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
with sodium hydroxide forms sodium hypobromite
Hypobromite
The hypobromite ion, also called alkaline bromine water, is BrO−. Bromine is in the +1 oxidation state. Hypobromite is the bromine compound analogous to hypochlorites found in common bleaches, and in immune cells...
in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
, which transforms the primary amide into an intermediate isocyanate
Isocyanate
Isocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
.

