
Mesogen
Encyclopedia
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
that induces structural order in the crystals.
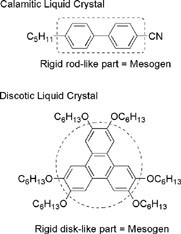
Typically, a liquid-crystalline molecule consists of a rigid moiety and one or more flexible parts. The rigid part aligns molecules in one direction, whereas the flexible parts induce fluidity in the liquid crystal. This rigid part is referred to as mesogen, and it plays a crucial role in the molecule. The optimum balance of these two parts is essential to form liquid-crystalline materials.
In a calamitic liquid crystal
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
, the mesogen is a rod-like structure composed of two or more aromatic and aliphatic rings connected in one direction. In a discotic liquid crystal
Columnar phase
The columnar phase is a class of mesophases in which molecules assemble into cylindrical structures to act as mesogens. Originally, these kinds of liquid crystals were called discotic liquid crystals because the columnar structures are composed of flat-shaped discotic molecules stacked...
, the flat-shaped aromatic core that makes molecules stack in one direction is defined as the mesogen.
These rod-like and disk-like structures are formed not only by covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s, but also by non-covalent interaction
Intermolecular force
Intermolecular forces are forces of attraction or repulsion which act between neighboring particles: atoms, molecules or ions. They are weak compared to the intramolecular forces, the forces which keep a molecule together...
s, such as hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s, ionic interaction
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
s, and metal coordination
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
. In such cases, key structures which define the macromolecular
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
shapes of the assembled molecules are called mesogens or mesogenic parts.
IUPAC
IUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
defines a "mesogen" according to its physico-chemical properties in the constitution of mesophase
Mesophase
In physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially-ordered structure in a mesophase...
, i.e. "liquid-crystal mesophase formation in low-molar-mass and polymeric substances"..

