Micellar cubic
Encyclopedia
A micellar cubic phase is a lyotropic liquid crystal
phase formed when the concentration of micelles dispersed in a solvent (usually water) is sufficiently high that they are forced to pack into a structure having long-ranged positional (translational) order. For example, spherical micelles a subic packing of a body-centred cubic lattice. Normal topology micellar cubic phases, denoted by the symbol I1, are the first lyotropic liquid crystalline phases that are formed by type I amphiphiles. The amphiphiles' hydrocarbon tails are contained on the inside of the micelle and hence the polar-apolar interface of the aggregates has a positive mean curvature
, by definition (it curves away from the polar phase). Inverse topology micellar cubic phases (such as the Fd3m phase) are observed for some type II amphiphiles at very high amphiphile concentrations. These aggregates, in which water is the minority phase, have a polar-apolar interface with a negative mean curvature
. The structures of the normal topology micellar cubic phases that ae formed by some types of amphiphiles (e.g. the oligoethyleneoxide monoalkyl ether series of non-ionic surfactants are the subject of debate. Micellar cubic phases are isotropic phases, but are distinguished from micellar solutions
by their very high viscosity. When thin film samples of micellar cubic phases are viewed under a polarising mcroscope they appear dark and featureless. Small air bubbles trapped in these preparations tend to appear highly distorted and occasionally have faceted surfaces.
Lyotropic liquid crystal
A liquid crystalline material is called lyotropic if phases having long-ranged orientational order are induced by the addition of a solvent. Historically the term was used to describe materials composed of amphiphilic molecules. Such molecules comprise a water-loving 'hydrophilic' head-group ...
phase formed when the concentration of micelles dispersed in a solvent (usually water) is sufficiently high that they are forced to pack into a structure having long-ranged positional (translational) order. For example, spherical micelles a subic packing of a body-centred cubic lattice. Normal topology micellar cubic phases, denoted by the symbol I1, are the first lyotropic liquid crystalline phases that are formed by type I amphiphiles. The amphiphiles' hydrocarbon tails are contained on the inside of the micelle and hence the polar-apolar interface of the aggregates has a positive mean curvature
Mean curvature
In mathematics, the mean curvature H of a surface S is an extrinsic measure of curvature that comes from differential geometry and that locally describes the curvature of an embedded surface in some ambient space such as Euclidean space....
, by definition (it curves away from the polar phase). Inverse topology micellar cubic phases (such as the Fd3m phase) are observed for some type II amphiphiles at very high amphiphile concentrations. These aggregates, in which water is the minority phase, have a polar-apolar interface with a negative mean curvature
Mean curvature
In mathematics, the mean curvature H of a surface S is an extrinsic measure of curvature that comes from differential geometry and that locally describes the curvature of an embedded surface in some ambient space such as Euclidean space....
. The structures of the normal topology micellar cubic phases that ae formed by some types of amphiphiles (e.g. the oligoethyleneoxide monoalkyl ether series of non-ionic surfactants are the subject of debate. Micellar cubic phases are isotropic phases, but are distinguished from micellar solutions
Micellar solutions
A micellar solution consists of a dispersion of micelles in a solvent . Micelles consist of aggregated amphiphiles, and in a micellar solution these are in equilibrium with free, unaggregated amphiphiles...
by their very high viscosity. When thin film samples of micellar cubic phases are viewed under a polarising mcroscope they appear dark and featureless. Small air bubbles trapped in these preparations tend to appear highly distorted and occasionally have faceted surfaces.
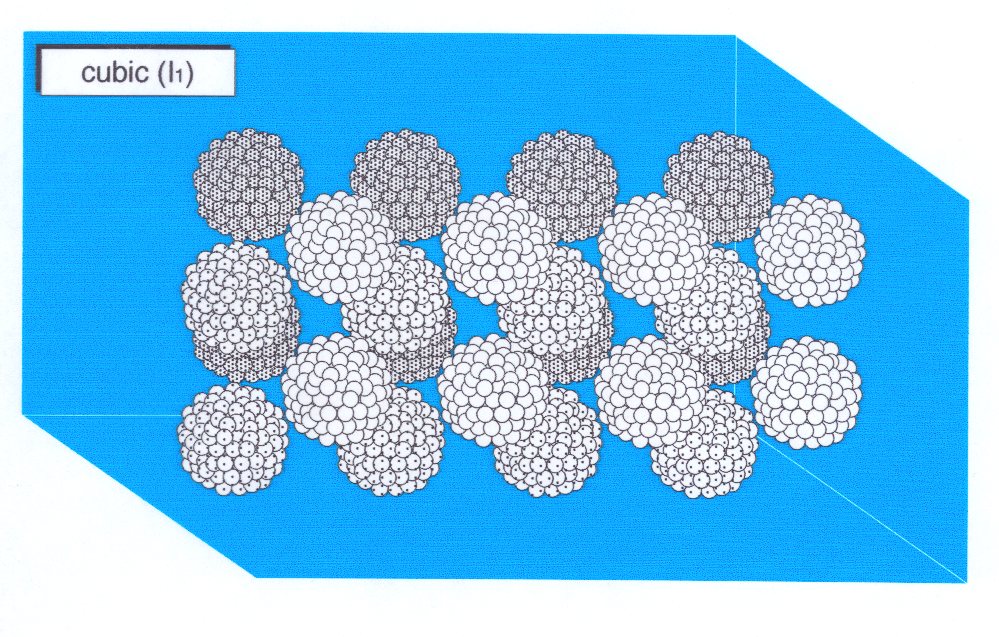 |
|---|
| Schematic of the structure of a type I micellar cubic phase showing spherical micelles disposed on a body-centred cubic lattice. |

