Active laser medium
Encyclopedia
The active laser medium (also called gain medium or lasing medium) is the source of optical gain
within a laser
. The gain results from the stimulated emission
of electronic or molecular transitions to a lower energy state from a higher energy state
previously populated by a pump source
.
Examples of active laser media include:
In order to lase, the active gain medium must be in a nonthermal energy distribution known as a population inversion
. The preparation of this state requires an external energy source and is known as laser pumping
. Pumping may be achieved with electrical currents (e.g. semiconductors, or gases via high-voltage discharges
) or with light, generated by discharge lamps or by other lasers (semiconductor lasers). More exotic gain media can be pumped by chemical reactions, nuclear fission
, or with high-energy electron beams.
 A universal model valid for all laser types does not exist.
A universal model valid for all laser types does not exist.
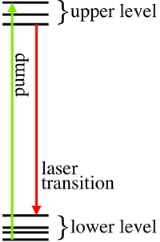
The simplest model includes two systems of sub-levels: upper and lower. Within each level, the fast transitions
lead to the Boltzmann distribution
of excitations among sub-levels (fig.1). The upper level is assumed to be metastable.
Also, gain and refractive index are assumed independent of a particular way of excitation.
For good performance of the gain medium, the separation between sub-levels should be larger than working temperature; then, at pump frequency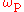 , the absorption dominates.
, the absorption dominates.
In the case of amplification
of optical signals, the lasing frequency is called signal frequency. However, the same term is used even in the laser oscillators, when amplified radiation is used to transfer energy rather than information. The model below seems to work well for most optically-pumped solid-state laser
s.
of absorption
and emission at frequencies and
and 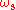 .
.
The relative concentrations can be defined as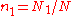 and
and  .
.
The rate of transitions of an active center from ground state to the excited state can be expressed with and
and
The rate of transitions back to the ground state can be expressed with ,
,
where and
and  are effective cross-sections
are effective cross-sections
of absorption at the frequencies of the pump and the signal.
 and
and  are the same for stimulated emission;
are the same for stimulated emission;
 is rate of the spontaneous decay of the upper level.
is rate of the spontaneous decay of the upper level.
Then, the kinetic equation for relative populations can be written as follows:
 ,
,
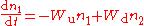
However, these equations keep .
.
The absorption at the pump frequency and the gain
at the pump frequency and the gain  at the signal frequency can be written
at the signal frequency can be written
as follows:
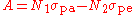 ,
,
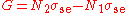 .
.
regime, causing the time derivative
s of populations to be negligible.
The steady-state solution can be written:
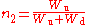 ,
,

The dynamic saturation intensities can be defined:
 ,
,
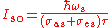 .
.
The absorption at strong signal:
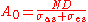 .
.
The gain at strong pump:
 ,
,
where
is determinant of cross-section.
Gain never exceeds value , and absorption never exceeds value
, and absorption never exceeds value 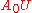 .
.
At given intensities ,
, 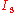 of pump and signal, the gain and absorption
of pump and signal, the gain and absorption
can be expressed as follows:
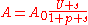 ,
,
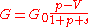 ,
,
where
 ,
,
 ,
,
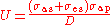 ,
,
 .
.
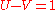 ,
, 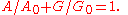
The state of gain medium can be characterized with a single parameter, such as population of the upper level, gain or absorption.
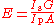 .
.
Within the same model, the efficiency can be expressed as follows:
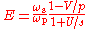 .
.
For the efficient operation both intensities, pump and signal should exceed their saturation intensities;
 , and
, and  .
.
The estimates above are valid for a medium uniformly filled with pump and signal light. The spatial hole burning may slightly reduce the efficiency because some regions are pumped well, but the pump is not efficiently withdrawn by the signal in the nodes of
the interference of counter-propagating waves.
Gain
In electronics, gain is a measure of the ability of a circuit to increase the power or amplitude of a signal from the input to the output. It is usually defined as the mean ratio of the signal output of a system to the signal input of the same system. It may also be defined on a logarithmic scale,...
within a laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
. The gain results from the stimulated emission
Stimulated emission
In optics, stimulated emission is the process by which an atomic electron interacting with an electromagnetic wave of a certain frequency may drop to a lower energy level, transferring its energy to that field. A photon created in this manner has the same phase, frequency, polarization, and...
of electronic or molecular transitions to a lower energy state from a higher energy state
previously populated by a pump source
Laser pumping
Laser pumping is the act of energy transfer from an external source into the gain medium of a laser. The energy is absorbed in the medium, producing excited states in its atoms. When the number of particles in one excited state exceeds the number of particles in the ground state or a less-excited...
.
Examples of active laser media include:
- Certain crystalCrystalA crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s, typically doped with rare-earthRare earth elementAs defined by IUPAC, rare earth elements or rare earth metals are a set of seventeen chemical elements in the periodic table, specifically the fifteen lanthanides plus scandium and yttrium...
ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s (e.g. neodymiumNeodymiumNeodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
, ytterbiumYtterbiumYtterbium is a chemical element with the symbol Yb and atomic number 70. A soft silvery metallic element, ytterbium is a rare earth element of the lanthanide series and is found in the minerals gadolinite, monazite, and xenotime. The element is sometimes associated with yttrium or other related...
, or erbiumErbiumErbium is a chemical element in the lanthanide series, with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements on Earth...
) or transition metalTransition metalThe term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
ions (titaniumTitaniumTitanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
or chromiumChromiumChromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
); most often yttrium aluminium garnetYttrium aluminium garnetYttrium aluminium garnet is a synthetic crystalline material of the garnet group. It is also one of three phases of the yttria-aluminium composite, the other two being yttrium aluminium monoclinic and yttrium aluminium perovskite . YAG is commonly used as a host material in various solid-state...
(YAG), yttrium orthovanadateYttrium orthovanadateYttrium orthovanadate is a transparent crystal. Undoped YVO4 is also used to make efficient high-power polarizing prisms similar to Glan–Taylor prisms.There are two principal applications for doped Yttrium orthovanadate:...
(YVO4), or sapphireSapphireSapphire is a gemstone variety of the mineral corundum, an aluminium oxide , when it is a color other than red or dark pink; in which case the gem would instead be called a ruby, considered to be a different gemstone. Trace amounts of other elements such as iron, titanium, or chromium can give...
(Al2O3); - GlassGlassGlass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
es, e.g. silicate or phosphate glasses, doped with laser-active ions; - GasGasGas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es, e.g. mixtures of heliumHeliumHelium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
and neonNeonNeon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
(HeNe), nitrogenNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, argonArgonArgon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
, carbon monoxideCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, or metal vapors; - SemiconductorSemiconductorA semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
s, e.g. gallium arsenide (GaAs), indium gallium arsenideIndium gallium arsenideIndium gallium arsenide is a semiconductor composed of indium, gallium and arsenic. It is used in high-power and high-frequency electronics because of its superior electron velocity with respect to the more common semiconductors silicon and gallium arsenide. InGaAs bandgap also makes it the...
(InGaAs), or gallium nitride (GaN). - Liquids, in the form of dye solutions as used in dye lasers.
In order to lase, the active gain medium must be in a nonthermal energy distribution known as a population inversion
Population inversion
In physics, specifically statistical mechanics, a population inversion occurs when a system exists in state with more members in an excited state than in lower energy states...
. The preparation of this state requires an external energy source and is known as laser pumping
Laser pumping
Laser pumping is the act of energy transfer from an external source into the gain medium of a laser. The energy is absorbed in the medium, producing excited states in its atoms. When the number of particles in one excited state exceeds the number of particles in the ground state or a less-excited...
. Pumping may be achieved with electrical currents (e.g. semiconductors, or gases via high-voltage discharges
Electric glow discharge
An electric glow discharge is a plasma formed by the passage of current at 100 V to several kV through a gas, often argon or another noble gas. It is found in products such as neon lamps and plasma-screen televisions, and is used in plasma physics and analytical chemistry.-Basic operating...
) or with light, generated by discharge lamps or by other lasers (semiconductor lasers). More exotic gain media can be pumped by chemical reactions, nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
, or with high-energy electron beams.
Example of a model of gain medium

The simplest model includes two systems of sub-levels: upper and lower. Within each level, the fast transitions
lead to the Boltzmann distribution
Boltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
of excitations among sub-levels (fig.1). The upper level is assumed to be metastable.
Also, gain and refractive index are assumed independent of a particular way of excitation.
For good performance of the gain medium, the separation between sub-levels should be larger than working temperature; then, at pump frequency
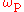 , the absorption dominates.
, the absorption dominates.In the case of amplification
Amplifier
Generally, an amplifier or simply amp, is a device for increasing the power of a signal.In popular use, the term usually describes an electronic amplifier, in which the input "signal" is usually a voltage or a current. In audio applications, amplifiers drive the loudspeakers used in PA systems to...
of optical signals, the lasing frequency is called signal frequency. However, the same term is used even in the laser oscillators, when amplified radiation is used to transfer energy rather than information. The model below seems to work well for most optically-pumped solid-state laser
Solid-state laser
A solid-state laser is a laser that uses a gain medium that is a solid, rather than a liquid such as in dye lasers or a gas as in gas lasers. Semiconductor-based lasers are also in the solid state, but are generally considered as a separate class from solid-state lasers .-Solid-state...
s.
Cross-sections
The simple medium can be characterized with effective cross-sectionsCross section (physics)
A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law....
of absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
and emission at frequencies
 and
and 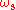 .
.
- Let
 be concentration of active centers in the solid-state lasers.
be concentration of active centers in the solid-state lasers. - Let
 be concentration of active centers in the ground state.
be concentration of active centers in the ground state. - Let
 be concentration of excited centers.
be concentration of excited centers. - Let
 .
.
The relative concentrations can be defined as
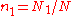 and
and  .
.The rate of transitions of an active center from ground state to the excited state can be expressed with
 and
andThe rate of transitions back to the ground state can be expressed with
 ,
,where
 and
and  are effective cross-sections
are effective cross-sectionsAbsorption cross section
Absorption cross section is a measure for the probability of an absorption process. More generally, the term cross section is used in physics to quantify the probability of a certain particle-particle interaction, e.g., scattering, electromagnetic absorption, etc...
of absorption at the frequencies of the pump and the signal.
 and
and  are the same for stimulated emission;
are the same for stimulated emission; is rate of the spontaneous decay of the upper level.
is rate of the spontaneous decay of the upper level.Then, the kinetic equation for relative populations can be written as follows:
 ,
,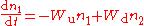
However, these equations keep
 .
.The absorption
 at the pump frequency and the gain
at the pump frequency and the gain  at the signal frequency can be written
at the signal frequency can be writtenas follows:
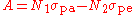 ,
,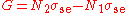 .
.Steady-state solution
In many cases the gain medium works in a continuous-wave or quasi-continuousQuasi-continuous function
In mathematics, the notion of a quasi-continuous function is similar to, but weaker than, the notion of a continuous function. All continuous functions are quasi-continuous but the converse is not true in general.-Definition:Let X be a topological space...
regime, causing the time derivative
Derivative
In calculus, a branch of mathematics, the derivative is a measure of how a function changes as its input changes. Loosely speaking, a derivative can be thought of as how much one quantity is changing in response to changes in some other quantity; for example, the derivative of the position of a...
s of populations to be negligible.
The steady-state solution can be written:
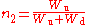 ,
,
The dynamic saturation intensities can be defined:
 ,
,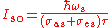 .
.The absorption at strong signal:
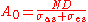 .
.The gain at strong pump:
 ,
,where

is determinant of cross-section.
Gain never exceeds value
 , and absorption never exceeds value
, and absorption never exceeds value 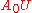 .
.At given intensities
 ,
, 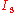 of pump and signal, the gain and absorption
of pump and signal, the gain and absorptioncan be expressed as follows:
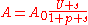 ,
,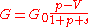 ,
,where
 ,
, ,
,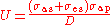 ,
, .
.Identities
The following identities take place: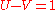 ,
, 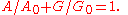
The state of gain medium can be characterized with a single parameter, such as population of the upper level, gain or absorption.
Efficiency of the gain medium
The efficiency of a gain medium can be defined as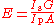 .
.Within the same model, the efficiency can be expressed as follows:
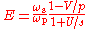 .
.For the efficient operation both intensities, pump and signal should exceed their saturation intensities;
 , and
, and  .
.The estimates above are valid for a medium uniformly filled with pump and signal light. The spatial hole burning may slightly reduce the efficiency because some regions are pumped well, but the pump is not efficiently withdrawn by the signal in the nodes of
the interference of counter-propagating waves.
See also
- Population inversionPopulation inversionIn physics, specifically statistical mechanics, a population inversion occurs when a system exists in state with more members in an excited state than in lower energy states...
- Laser constructionLaser constructionA laser is constructed from three principal parts:*An energy source ,*A gain medium or laser medium, and*Two or more mirrors that form an optical resonator.-Pump source:...
- Laser scienceLaser scienceLaser science or laser physics is a branch of optics that describes the theory and practice of lasers.Laser science is principally concerned with quantum electronics, laser construction, optical cavity design, the physics of producing a population inversion in laser media, and the temporal...
- List of laser types
External links
- Gain media Encyclopedia of Laser Physics and Technology

