
Isotopes of plutonium
Encyclopedia
Plutonium
(Pu) is an artificial element, except for trace quantities of primordial
244Pu
, and thus a standard atomic mass
cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope
synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are Pu-244
, with a half-life
of 80.8 million years, Pu-242, with a half-life of 373,300 years, and Pu-239
, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states, though none are very stable (all have half-lives less than one second).
The isotopes of plutonium range in atomic weight
from 228.0387 u
(Pu-228) to 247.074 u (Pu-247). The primary decay modes before the most stable isotope, Pu-244
, are spontaneous fission
and alpha emission; the intial mode after is beta emission. The primary decay product
s before Pu-244 are isotopes of uranium
and neptunium
(neglecting the wide range of daughter nuclei created by fission processes), and the primary products after are isotopes of americium
.

 Pu-239, a fissile
Pu-239, a fissile
isotope which is the second most used nuclear fuel
in nuclear reactor
s after U-235
, and the most used fuel in the fission
portion of nuclear weapons, is produced from U-238
by neutron capture
followed by two beta decay
s.
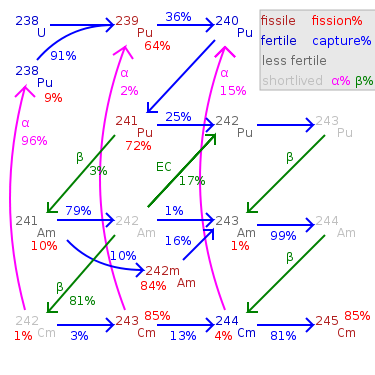
Pu-240, Pu-241, Pu-242 are produced by further neutron capture. The odd-mass isotopes Pu-239 and Pu-241 have about a 3/4 chance of undergoing fission
on capture of a thermal neutron and about a 1/4 chance of retaining the neutron
and becoming the following isotope. The even-mass isotopes are fertile material
but not fissile
and also have a lower overall probability (cross section
) of neutron capture; therefore, they tend to accumulate in nuclear fuel
used in a thermal reactor
, the design of all nuclear power plant
s today. In plutonium that has been used a second time in thermal reactors in MOX fuel
, Pu-240 may even be the most common isotope. All plutonium isotopes and other actinides, however, are fissionable with fast neutrons. Pu-240 does have a moderate thermal neutron absorption cross section, so that Pu-241 production in a thermal reactor becomes a significant fraction as large as Pu-239 production.
Pu-241 has a halflife of 14 years, and has slightly higher thermal neutron cross sections than Pu-239 for both fission and absorption. While nuclear fuel is being used in a reactor, a Pu-241 nucleus is much more likely to fission or to capture a neutron than to decay. Pu-241 accounts for a significant proportion of fissions in thermal reactor fuel that has been used for some time. However, in spent nuclear fuel
that does not quickly undergo nuclear reprocessing
but instead is cooled for years after use, much or most of the Pu-241 will beta decay to americium
-241, one of the minor actinides
, a strong alpha emitter, and difficult to use in thermal reactors.
Pu-242 has a particularly low cross section for thermal neutron capture; and it takes four neutron absorptions to become another fissile isotope (either curium
-245 or Pu-241) and fission. Even then, there is a chance either of those two fissile isotopes will fail to fission but instead absorb the fourth neutron, becoming curium-246 (on the way to even heavier actinides like californium
, which is a neutron emitter by spontaneous fission and difficult to handle) or becoming Pu-242 again; so the mean number of neutrons absorbed before fission is even higher than 4. Therefore Pu-242 is particularly unsuited to recycling in a thermal reactor
and would be better used in a fast reactor where it can be fissioned directly. However, Pu-242's low cross section means that relatively little of it will be transmuted during one cycle in a thermal reactor. Pu-242's halflife is about 15 times as long as Pu-239's halflife; therefore it is 1/15 as radioactive and not one of the larger contributors to nuclear waste radioactivity.
242Pu's gamma ray
emissions are also weaker than those of the other isotopes.
Pu-243 has a halflife of only 5 hours, beta decaying to americium
-243. Because Pu-243 has little opportunity to capture an additional neutron before decay, the nuclear fuel cycle
does not produce the extremely long-lived Pu-244 in significant quantity.
Pu-238 is not normally produced in as large quantity by the nuclear fuel cycle, but some is produced from neptunium-237 by neutron capture (this reaction can also be used with purified neptunium to produce Pu-238 relatively free of other plutonium isotopes for use in radioisotope thermoelectric generators), by the (n,2n) reaction of fast neutrons on Pu-239, or by alpha decay of curium
-242 which is produced by neutron capture from Am-241. It has significant thermal neutron cross section for fission, but is more likely to capture a neutron and become Pu-239.
for 239Pu is 270 barns
, while the fission cross section is 747 barns for thermal neutrons. The higher plutonium isotopes are created when the uranium fuel is used for a long time. It is the case that for high burnup used fuel that the concentrations of the higher plutonium isotopes will be higher than the low burnup fuel which is reprocessed to obtain weapons grade plutonium.
materials used for the production of nuclear weapon
s and in some nuclear reactor
s as a source of energy. The other fissile materials are uranium-235
and uranium-233
. Plutonium-239 is virtually nonexistent in nature. It is made by bombarding uranium-238
with neutrons in a nuclear reactor. Uranium-238 is present in quantity in most reactor fuel; hence plutonium-239 is continuously made in these reactors. Since plutonium-239 can itself be split by neutron
s to release energy, plutonium-239 provides a portion of the energy generation in a nuclear reactor.

-237. Since nearly all neptunium is produced in this way or consists of isotopes which decay quickly, one gets nearly pure Np-237 by chemical separation of neptunium. After this chemical separation, Np-237 is again irradiated by reactor neutrons to be converted to Np-238 which decays to Pu-238 with a half-life of 2 days.
as a secondary decay mode at a small but significant rate. The presence of Pu-240 limits the plutonium's nuclear bomb potential because the neutron flux from spontaneous fission, initiates the chain reaction
prematurely and reduces the bomb's power by exploding the core before full implosion
is reached. Plutonium consisting of more than about 90% Pu-239 is called weapons-grade plutonium; plutonium from spent nuclear fuel
from commercial power reactors generally contains at least 20% Pu-240 and is called reactor-grade plutonium. However, modern nuclear weapons use fusion boosting which mitigates the predetonation problem; if the pit
can generate a nuclear weapon yield
of even a fraction of a kiloton, which is enough to start deuterium-tritium fusion
, the resulting burst of neutrons will fission enough plutonium to ensure a yield of tens of kilotons.
Pu-240 contamination is the reason plutonium weapons must use the implosion method. Theoretically, pure Pu-239 could be used in a gun-type nuclear weapon, but achieving this level of purity is prohibitively difficult. Pu-240 contamination has proven a mixed blessing to nuclear weapons design. While it created delays and headaches during the Manhattan Project
because of the need to develop implosion technology, those very same difficulties are currently a barrier to nuclear proliferation
. Implosion devices are also inherently more efficient and less prone toward accidental detonation than are gun-type weapons.
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
(Pu) is an artificial element, except for trace quantities of primordial
Primordial nuclide
In geochemistry and geonuclear physics, primordial nuclides or primordial isotopes are nuclides found on the earth that have existed in their current form since before Earth was formed. Only 288 such nuclides are known...
244Pu
Plutonium-244
Plutonium-244 is an isotope of plutonium that has a halflife of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any actinide except for the three naturally abundant ones uranium-235 , uranium-238, and thorium-232...
, and thus a standard atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are Pu-244
Plutonium-244
Plutonium-244 is an isotope of plutonium that has a halflife of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any actinide except for the three naturally abundant ones uranium-235 , uranium-238, and thorium-232...
, with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 80.8 million years, Pu-242, with a half-life of 373,300 years, and Pu-239
Plutonium-239
Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states, though none are very stable (all have half-lives less than one second).
The isotopes of plutonium range in atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
from 228.0387 u
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
(Pu-228) to 247.074 u (Pu-247). The primary decay modes before the most stable isotope, Pu-244
Plutonium-244
Plutonium-244 is an isotope of plutonium that has a halflife of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any actinide except for the three naturally abundant ones uranium-235 , uranium-238, and thorium-232...
, are spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
and alpha emission; the intial mode after is beta emission. The primary decay product
Decay product
In nuclear physics, a decay product is the remaining nuclide left over from radioactive decay. Radioactive decay often involves a sequence of steps...
s before Pu-244 are isotopes of uranium
Isotopes of uranium
Uranium is a naturally occurring radioactive element that has no stable isotopes but two primordial isotopes that have long half-life and are found in appreciable quantity in the Earth's crust, along with the decay product uranium-234. The average atomic mass of natural uranium is 238.02891 u...
and neptunium
Isotopes of neptunium
Neptunium is an artificial element, and thus a standard atomic mass cannot be given. Like all artificial elements, it has no stable isotopes...
(neglecting the wide range of daughter nuclei created by fission processes), and the primary products after are isotopes of americium
Isotopes of americium
Americium is an artificial element, and thus a standard atomic mass cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 241Am in 1944....
.
Notable Isotopes
- Plutonium-238Plutonium-238-External links:**...
has a half-life of 87.74 years and emits alpha particleAlpha particleAlpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s. Pure Pu-238 for radioisotope thermoelectric generatorRadioisotope thermoelectric generatorA radioisotope thermoelectric generator is an electrical generator that obtains its power from radioactive decay. In such a device, the heat released by the decay of a suitable radioactive material is converted into electricity by the Seebeck effect using an array of thermocouples.RTGs can be...
s which power some spacecraftSpacecraftA spacecraft or spaceship is a craft or machine designed for spaceflight. Spacecraft are used for a variety of purposes, including communications, earth observation, meteorology, navigation, planetary exploration and transportation of humans and cargo....
is produced by neutron capture on neptunium-237 but plutonium from spent nuclear fuelSpent nuclear fuelSpent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor...
can contain as much as a few percent of Pu-238, from either 237Np, alpha decayAlpha decayAlpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
of 242Cm, or (n,2n) reactions. - Plutonium-239Plutonium-239Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
is the most important isotopeIsotopeIsotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of plutonium, with a half-lifeHalf-lifeHalf-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 24,100 years. Pu-239 and Pu-241 are fissileFissileIn nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
, meaning that the nuclei of its atoms can break apartNuclear fissionIn nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
by being bombarded by slow movingNeutron temperatureThe neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is...
thermal neutrons, releasing energy, gamma radiation and more neutronsNeutron radiationNeutron radiation is a kind of ionizing radiation which consists of free neutrons. A result of nuclear fission or nuclear fusion, it consists of the release of free neutrons from atoms, and these free neutrons react with nuclei of other atoms to form new isotopes, which, in turn, may produce...
. It can therefore sustain a nuclear chain reactionNuclear chain reactionA nuclear chain reaction occurs when one nuclear reaction causes an average of one or more nuclear reactions, thus leading to a self-propagating number of these reactions. The specific nuclear reaction may be the fission of heavy isotopes or the fusion of light isotopes...
, leading to applications in nuclear weaponNuclear weaponA nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s and nuclear reactorNuclear reactorA nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s. Pu-239 is synthesized by irradiating uranium-238Uranium-238Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
with neutrons in a nuclear reactorNuclear reactorA nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
, then recovered via nuclear reprocessingNuclear reprocessingNuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
of the fuel. Further neutron captureNeutron captureNeutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
produces successively heavier isotopes. - Plutonium-240Plutonium-240Plutonium-240 is an isotope of the metal plutonium formed when plutonium-239 captures a neutron. About 62% to 73% of the time when Pu-239 captures a neutron it undergoes fission; the rest of the time it forms Pu-240. The longer a nuclear fuel element remains in a nuclear reactor the greater the...
has a high rate of spontaneous fissionSpontaneous fissionSpontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
, raising the background neutron radiationNeutron radiationNeutron radiation is a kind of ionizing radiation which consists of free neutrons. A result of nuclear fission or nuclear fusion, it consists of the release of free neutrons from atoms, and these free neutrons react with nuclei of other atoms to form new isotopes, which, in turn, may produce...
of plutonium containing it. Plutonium is graded by proportion of Pu-240: weapons grade (< 7%), fuel grade (7–19%) and reactor grade (> 19%). Lower grades are less suited for nuclear weaponNuclear weaponA nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s and thermal reactorThermal reactorA thermal reactor is a nuclear reactor that uses slow or thermal neutrons. Most power reactors are of this type. These type of reactors use a neutron moderator to slow neutrons until they approach the average kinetic energy of the surrounding particles, that is, to reduce the speed of the neutrons...
s but can fuel fast reactors. - Plutonium-241Plutonium-241Plutonium-241 is an isotope of plutonium formed when plutonium-240 captures a neutron. Like Pu-239 but unlike 240Pu, 241Pu is fissile, with a neutron absorption cross section about 1/3 greater than 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission...
is fissile, but also beta decayBeta decayIn nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
s with a halflife of 14 years to americium-241. - Plutonium-242Plutonium-242Pu-242 is one of the isotopes of plutonium, the second longest-lived, with a half-life of 373,300 years.242Pu's halflife is about 15 times as long as Pu-239's halflife; therefore it is 1/15 as radioactive and not one of the larger contributors to nuclear waste radioactivity.242Pu's gamma ray...
is not fissile, not very fertile (requiring 3 more neutron captures to become fissile), has a low neutron capture cross sectionNeutron cross-sectionIn nuclear and particle physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power...
, and a longer halflife than any of the lighter isotopes. - Plutonium-244Plutonium-244Plutonium-244 is an isotope of plutonium that has a halflife of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any actinide except for the three naturally abundant ones uranium-235 , uranium-238, and thorium-232...
is the most stable isotope of plutonium, with a half-life of about 80 million years, long enough to be found in trace quantities in nature. It is not significantly produced in nuclear reactors because Pu-243 has a short halflife, but some is produced in nuclear explosions.
Production and uses


Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
isotope which is the second most used nuclear fuel
Nuclear fuel
Nuclear fuel is a material that can be 'consumed' by fission or fusion to derive nuclear energy. Nuclear fuels are the most dense sources of energy available...
in nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s after U-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
, and the most used fuel in the fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
portion of nuclear weapons, is produced from U-238
Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
by neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
followed by two beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
s.
Pu-240, Pu-241, Pu-242 are produced by further neutron capture. The odd-mass isotopes Pu-239 and Pu-241 have about a 3/4 chance of undergoing fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
on capture of a thermal neutron and about a 1/4 chance of retaining the neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
and becoming the following isotope. The even-mass isotopes are fertile material
Fertile material
Fertile material is a term used to describe nuclides which generally themselves do not undergo induced fission but from which fissile material is generated by neutron absorption and subsequent nuclei conversions...
but not fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
and also have a lower overall probability (cross section
Neutron cross-section
In nuclear and particle physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power...
) of neutron capture; therefore, they tend to accumulate in nuclear fuel
Nuclear fuel
Nuclear fuel is a material that can be 'consumed' by fission or fusion to derive nuclear energy. Nuclear fuels are the most dense sources of energy available...
used in a thermal reactor
Thermal reactor
A thermal reactor is a nuclear reactor that uses slow or thermal neutrons. Most power reactors are of this type. These type of reactors use a neutron moderator to slow neutrons until they approach the average kinetic energy of the surrounding particles, that is, to reduce the speed of the neutrons...
, the design of all nuclear power plant
Nuclear power plant
A nuclear power plant is a thermal power station in which the heat source is one or more nuclear reactors. As in a conventional thermal power station the heat is used to generate steam which drives a steam turbine connected to a generator which produces electricity.Nuclear power plants are usually...
s today. In plutonium that has been used a second time in thermal reactors in MOX fuel
MOX fuel
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material. MOX fuel contains plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the...
, Pu-240 may even be the most common isotope. All plutonium isotopes and other actinides, however, are fissionable with fast neutrons. Pu-240 does have a moderate thermal neutron absorption cross section, so that Pu-241 production in a thermal reactor becomes a significant fraction as large as Pu-239 production.
Pu-241 has a halflife of 14 years, and has slightly higher thermal neutron cross sections than Pu-239 for both fission and absorption. While nuclear fuel is being used in a reactor, a Pu-241 nucleus is much more likely to fission or to capture a neutron than to decay. Pu-241 accounts for a significant proportion of fissions in thermal reactor fuel that has been used for some time. However, in spent nuclear fuel
Spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor...
that does not quickly undergo nuclear reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
but instead is cooled for years after use, much or most of the Pu-241 will beta decay to americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
-241, one of the minor actinides
Minor actinides
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium, americium, curium, berkelium, californium, einsteinium, and fermium...
, a strong alpha emitter, and difficult to use in thermal reactors.
Pu-242 has a particularly low cross section for thermal neutron capture; and it takes four neutron absorptions to become another fissile isotope (either curium
Curium
Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
-245 or Pu-241) and fission. Even then, there is a chance either of those two fissile isotopes will fail to fission but instead absorb the fourth neutron, becoming curium-246 (on the way to even heavier actinides like californium
Californium
Californium is a radioactive metallic chemical element with the symbol Cf and atomic number 98. The element was first made in the laboratory in 1950 by bombarding curium with alpha particles at the University of California, Berkeley. It is the ninth member of the actinide series and was the...
, which is a neutron emitter by spontaneous fission and difficult to handle) or becoming Pu-242 again; so the mean number of neutrons absorbed before fission is even higher than 4. Therefore Pu-242 is particularly unsuited to recycling in a thermal reactor
Thermal reactor
A thermal reactor is a nuclear reactor that uses slow or thermal neutrons. Most power reactors are of this type. These type of reactors use a neutron moderator to slow neutrons until they approach the average kinetic energy of the surrounding particles, that is, to reduce the speed of the neutrons...
and would be better used in a fast reactor where it can be fissioned directly. However, Pu-242's low cross section means that relatively little of it will be transmuted during one cycle in a thermal reactor. Pu-242's halflife is about 15 times as long as Pu-239's halflife; therefore it is 1/15 as radioactive and not one of the larger contributors to nuclear waste radioactivity.
242Pu's gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
emissions are also weaker than those of the other isotopes.
Pu-243 has a halflife of only 5 hours, beta decaying to americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
-243. Because Pu-243 has little opportunity to capture an additional neutron before decay, the nuclear fuel cycle
Nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in...
does not produce the extremely long-lived Pu-244 in significant quantity.
Pu-238 is not normally produced in as large quantity by the nuclear fuel cycle, but some is produced from neptunium-237 by neutron capture (this reaction can also be used with purified neptunium to produce Pu-238 relatively free of other plutonium isotopes for use in radioisotope thermoelectric generators), by the (n,2n) reaction of fast neutrons on Pu-239, or by alpha decay of curium
Curium
Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
-242 which is produced by neutron capture from Am-241. It has significant thermal neutron cross section for fission, but is more likely to capture a neutron and become Pu-239.
Pu-240, Pu-241 and Pu-242
The activation cross sectionCross section (physics)
A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law....
for 239Pu is 270 barns
Barn (unit)
A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the...
, while the fission cross section is 747 barns for thermal neutrons. The higher plutonium isotopes are created when the uranium fuel is used for a long time. It is the case that for high burnup used fuel that the concentrations of the higher plutonium isotopes will be higher than the low burnup fuel which is reprocessed to obtain weapons grade plutonium.
| Isotope | cross section Cross section (physics) A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law.... | decay mode | 238U Uranium-238 Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239... | 2.7 | α | 4.47 x 109 years |
|---|---|---|---|---|
| 239U | β | 23 minutes | ||
| 239Np | β | 2.36 days | ||
| 239Pu Plutonium-239 Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in... |
270 | α | 24,110 years | |
| 240Pu Plutonium-240 Plutonium-240 is an isotope of the metal plutonium formed when plutonium-239 captures a neutron. About 62% to 73% of the time when Pu-239 captures a neutron it undergoes fission; the rest of the time it forms Pu-240. The longer a nuclear fuel element remains in a nuclear reactor the greater the... |
289 | α | 6,564 years | |
| 241Pu Plutonium-241 Plutonium-241 is an isotope of plutonium formed when plutonium-240 captures a neutron. Like Pu-239 but unlike 240Pu, 241Pu is fissile, with a neutron absorption cross section about 1/3 greater than 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission... |
362 | β | 14.35 years | |
| 242Pu Plutonium-242 Pu-242 is one of the isotopes of plutonium, the second longest-lived, with a half-life of 373,300 years.242Pu's halflife is about 15 times as long as Pu-239's halflife; therefore it is 1/15 as radioactive and not one of the larger contributors to nuclear waste radioactivity.242Pu's gamma ray... |
18.8 | α | 373,300 years |
Pu-239
Plutonium-239 is one of the three fissileFissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
materials used for the production of nuclear weapon
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s and in some nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s as a source of energy. The other fissile materials are uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
and uranium-233
Uranium-233
Uranium-233 is a fissile isotope of uranium, bred from Thorium as part of the thorium fuel cycle. It has been used in a few nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years....
. Plutonium-239 is virtually nonexistent in nature. It is made by bombarding uranium-238
Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
with neutrons in a nuclear reactor. Uranium-238 is present in quantity in most reactor fuel; hence plutonium-239 is continuously made in these reactors. Since plutonium-239 can itself be split by neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s to release energy, plutonium-239 provides a portion of the energy generation in a nuclear reactor.

| Element | Isotope | Thermal neutron capture cross section Cross section (physics) A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law.... (barn Barn (unit) A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the... ) | Thermal neutron fission Cross section (barn Barn (unit) A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the... ) | decay mode | halflife |
|---|---|---|---|---|---|
| U Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
238 | 2.68 | 5·10−6 | α | 4.47 x 109 years |
| U Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
239 | 22 | 15 | β | 23 minutes |
| Np Neptunium Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a... |
239 | 30 | 1 | β | 2.36 days |
| Pu Plutonium Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation... |
239 | 271 | 750 | α | 24,110 years |
Pu-238
There are small amounts of Pu-238 in the plutonium of usual plutonium-producing reactors. However, isotopic separation would be quite expensive compared to another method: when a U-235 atom captures a neutron, it is converted to an excited state of U-236. Some of the excited U-236 nuclei undergo fission, but some decay to the ground state of U-236 by emitting gamma radiation. Further neutron capture creates U-237 which has a half-life of 7 days and thus quickly decays to NpNeptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
-237. Since nearly all neptunium is produced in this way or consists of isotopes which decay quickly, one gets nearly pure Np-237 by chemical separation of neptunium. After this chemical separation, Np-237 is again irradiated by reactor neutrons to be converted to Np-238 which decays to Pu-238 with a half-life of 2 days.
| Element | Isotope | Thermal neutron cross section Cross section (physics) A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law.... | decay mode | halflife |
|---|---|---|---|---|
| U Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
235 | 99 | α | 703,800,000 years |
| U Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
236 | 5.3 | α | 23,420,000 years |
| U Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
237 | - | β | 6.75 days |
| Np Neptunium Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a... |
237 | 165 (capture) | α | 2,144,000 years |
| Np Neptunium Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a... |
238 | - | β | 2.11 days |
| Pu Plutonium Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation... |
238 | - | α | 87.7 years |
Pu-240 as obstacle to nuclear weapons
Pu-240 undergoes spontaneous fissionSpontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
as a secondary decay mode at a small but significant rate. The presence of Pu-240 limits the plutonium's nuclear bomb potential because the neutron flux from spontaneous fission, initiates the chain reaction
Chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events....
prematurely and reduces the bomb's power by exploding the core before full implosion
Implosion
Implosion is a process in which objects are destroyed by collapsing on themselves. The opposite of explosion, implosion concentrates matter and energy....
is reached. Plutonium consisting of more than about 90% Pu-239 is called weapons-grade plutonium; plutonium from spent nuclear fuel
Spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor...
from commercial power reactors generally contains at least 20% Pu-240 and is called reactor-grade plutonium. However, modern nuclear weapons use fusion boosting which mitigates the predetonation problem; if the pit
Pit (nuclear weapon)
The pit is the core of an implosion weapon – the fissile material and any neutron reflector or tamper bonded to it. Some weapons tested during the 1950s used pits made with U-235 alone, or in composite with plutonium, but all-plutonium pits are the smallest in diameter and have been the standard...
can generate a nuclear weapon yield
Nuclear weapon yield
The explosive yield of a nuclear weapon is the amount of energy discharged when a nuclear weapon is detonated, expressed usually in the equivalent mass of trinitrotoluene , either in kilotons or megatons , but sometimes also in terajoules...
of even a fraction of a kiloton, which is enough to start deuterium-tritium fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
, the resulting burst of neutrons will fission enough plutonium to ensure a yield of tens of kilotons.
Pu-240 contamination is the reason plutonium weapons must use the implosion method. Theoretically, pure Pu-239 could be used in a gun-type nuclear weapon, but achieving this level of purity is prohibitively difficult. Pu-240 contamination has proven a mixed blessing to nuclear weapons design. While it created delays and headaches during the Manhattan Project
Manhattan Project
The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army...
because of the need to develop implosion technology, those very same difficulties are currently a barrier to nuclear proliferation
Nuclear proliferation
Nuclear proliferation is a term now used to describe the spread of nuclear weapons, fissile material, and weapons-applicable nuclear technology and information, to nations which are not recognized as "Nuclear Weapon States" by the Treaty on the Nonproliferation of Nuclear Weapons, also known as the...
. Implosion devices are also inherently more efficient and less prone toward accidental detonation than are gun-type weapons.
Table
| nuclide symbol |
Z(p Proton The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.... ) |
N(n Neutron The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of... ) |
isotopic mass (u) |
half-life | decay mode(s)Abbreviations: CD: Cluster decay Cluster decay Cluster decay is a type of nuclear decay in which a parent atomic nucleus with A nucleons and Z protons emits a cluster of Ne neutrons and Ze protons heavier than an alpha particle but lighter than a typical binary fission fragment Cluster decay (also named heavy particle radioactivity or heavy... EC: Electron capture Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... IT: Isomeric transition Isomeric transition An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer.... SF: Spontaneous fission Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... |
daughter isotopesBold for stable isotopes |
nuclear spin |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|
| excitation energy | |||||||||
| 228Pu | 94 | 134 | 228.03874(3) | 1.1(+20-5) s | α (99.9%) | 224U | 0+ | ||
| β+ Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... (.1%) |
228Np | ||||||||
| 229Pu | 94 | 135 | 229.04015(6) | 120(50) s | α | 225U | 3/2+# | ||
| 230Pu | 94 | 136 | 230.039650(16) | 1.70(17) min | α | 226U | 0+ | ||
| β+ (rare) | 230Np | ||||||||
| 231Pu | 94 | 137 | 231.041101(28) | 8.6(5) min | β+ | 231Np | 3/2+# | ||
| α (rare) | 227U | ||||||||
| 232Pu | 94 | 138 | 232.041187(19) | 33.7(5) min | EC Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... (89%) |
232Np | 0+ | ||
| α (11%) | 228U | ||||||||
| 233Pu | 94 | 139 | 233.04300(5) | 20.9(4) min | β+ (99.88%) | 233Np | 5/2+# | ||
| α (.12%) | 229U | ||||||||
| 234Pu | 94 | 140 | 234.043317(7) | 8.8(1) h | EC (94%) | 234Np | 0+ | ||
| α (6%) | 230U | ||||||||
| 235Pu | 94 | 141 | 235.045286(22) | 25.3(5) min | β+ (99.99%) | 235Np | (5/2+) | ||
| α (.0027%) | 231U | ||||||||
| 236Pu | 94 | 142 | 236.0460580(24) | 2.858(8) a | α | 232U | 0+ | ||
| SF Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... (1.37×10−7%) |
(various) | ||||||||
| CD Cluster decay Cluster decay is a type of nuclear decay in which a parent atomic nucleus with A nucleons and Z protons emits a cluster of Ne neutrons and Ze protons heavier than an alpha particle but lighter than a typical binary fission fragment Cluster decay (also named heavy particle radioactivity or heavy... (2×10−12%) |
208Pb 28Mg |
||||||||
| β+β+ (rare) | 236U | ||||||||
| 237Pu | 94 | 143 | 237.0484097(24) | 45.2(1) d | EC | 237Np | 7/2- | ||
| α (.0042%) | 233U | ||||||||
| 237m1Pu | 145.544(10) keV | 180(20) ms | IT Isomeric transition An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer.... |
237Pu | 1/2+ | ||||
| 237m2Pu | 2900(250) keV | 1.1(1) µs | |||||||
| 238Pu Plutonium-238 -External links:**... |
94 | 144 | 238.0495599(20) | 87.7(1) a | α | 234U | 0+ | ||
| SF (1.9×10−7%) | (various) | ||||||||
| CD (1.4×10−14%) | 206Hg 32Si |
||||||||
| CD (6×10−15%) | 180Yb 30Mg 28Mg |
||||||||
| 239Pu Plutonium-239 Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in... Fissile Fissile In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons... nuclideMost useful isotope for nuclear weapon Nuclear weapon A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount... s |
94 | 145 | 239.0521634(20) | 2.411(3)×104 a | α | 235mU | 1/2+ | ||
| SF (3.1×10−10%) | (various) | ||||||||
| 239m1Pu | 391.584(3) keV | 193(4) ns | 7/2- | ||||||
| 239m2Pu | 3100(200) keV | 7.5(10) µs | (5/2+) | ||||||
| 240Pu Plutonium-240 Plutonium-240 is an isotope of the metal plutonium formed when plutonium-239 captures a neutron. About 62% to 73% of the time when Pu-239 captures a neutron it undergoes fission; the rest of the time it forms Pu-240. The longer a nuclear fuel element remains in a nuclear reactor the greater the... |
94 | 146 | 240.0538135(20) | 6,561(7) a | α | 236U | 0+ | ||
| SF (5.7×10−6%) | (various) | ||||||||
| CD (1.3×10−13%) | 206Hg 34Si |
||||||||
| 241Pu Plutonium-241 Plutonium-241 is an isotope of plutonium formed when plutonium-240 captures a neutron. Like Pu-239 but unlike 240Pu, 241Pu is fissile, with a neutron absorption cross section about 1/3 greater than 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission... |
94 | 147 | 241.0568515(20) | 14.290(6) a | β- (99.99%) | 241Am | 5/2+ | ||
| α (.00245%) | 237U | ||||||||
| SF (2.4×10−14%) | (various) | ||||||||
| 241m1Pu | 161.6(1) keV | 0.88(5) µs | 1/2+ | ||||||
| 241m2Pu | 2200(200) keV | 21(3) µs | |||||||
| 242Pu Plutonium-242 Pu-242 is one of the isotopes of plutonium, the second longest-lived, with a half-life of 373,300 years.242Pu's halflife is about 15 times as long as Pu-239's halflife; therefore it is 1/15 as radioactive and not one of the larger contributors to nuclear waste radioactivity.242Pu's gamma ray... |
94 | 148 | 242.0587426(20) | 3.75(2)×105 a | α | 238U | 0+ | ||
| SF (5.5×10−4%) | (various) | ||||||||
| 243Pu | 94 | 149 | 243.062003(3) | 4.956(3) h | β- | 243Am | 7/2+ | ||
| 243mPu | 383.6(4) keV | 330(30) ns | (1/2+) | ||||||
| 244Pu Plutonium-244 Plutonium-244 is an isotope of plutonium that has a halflife of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any actinide except for the three naturally abundant ones uranium-235 , uranium-238, and thorium-232... Primordial Primordial nuclide In geochemistry and geonuclear physics, primordial nuclides or primordial isotopes are nuclides found on the earth that have existed in their current form since before Earth was formed. Only 288 such nuclides are known... radionuclide Radionuclide A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma... |
94 | 150 | 244.064204(5) | 8.00(9)×107 a | α (99.88%) | 240U | 0+ | Trace | |
| SF (.123%) | (various) | ||||||||
| β-β- (7.3×10−9%) | 244Cm | ||||||||
| 245Pu | 94 | 151 | 245.067747(15) | 10.5(1) h | β- | 245Am | (9/2-) | ||
| 246Pu | 94 | 152 | 246.070205(16) | 10.84(2) d | β- | 246mAm | 0+ | ||
| 247Pu | 94 | 153 | 247.07407(32)# | 2.27(23) d | β- | 247Am | 1/2+# | ||

