
Implicit solvation
Encyclopedia
Implicit solvation is a method of representing solvent
as a continuous medium instead of individual “explicit” solvent molecules most often used in molecular dynamics
simulations and in other applications of molecular mechanics
. The method is often applied to estimate free energy
of solute
-solvent
interactions in structural and chemical processes, such as folding or conformational transitions
of proteins, DNA
, RNA
, and polysaccharide
s, association of biological macromolecules with ligand
s, or transport of drugs
across biological membrane
s.
The implicit solvation model is justified in liquids, where the potential of mean force
can be applied to approximate the averaged behavior of many highly dynamic solvent molecules. However, the interiors of biological membrane
s or protein
s can also be considered as media with specific solvation
or dielectric
properties. These media are continuous but not necessarily uniform, since their properties can be described by different analytical functions, such as “polarity profiles” of lipid bilayer
s. There are two basic types of implicit solvent methods: models based on accessible surface area
s (ASA) that were historically the first, and more recent continuum electrostatics models, although various modifications and combinations of the different methods are possible.
The accessible surface area
(ASA) method is based on experimental linear relations between Gibbs free energy
of transfer and the surface area
of a solute
molecule. This method operates directly with free energy of solvation
, unlike molecular mechanics
or electrostatic methods that include only the enthalpic
component of free energy. The continuum representation of solvent also significantly improves the computational speed and reduces errors in statistical averaging that arise from incomplete sampling of solvent conformations, so that the energy landscapes obtained with implicit and explicit solvent are different. Although the implicit solvent model is useful for simulations of biomolecules, this is an approximate method with certain limitations and problems related to parameterization and treatment of ionization
effects.
molecule in the simplest ASA-based method is given by: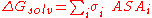
where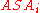 is accessible surface area
is accessible surface area
of atom i, and
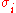 is solvation parameter of atom i, i.e. a contribution to the free energy of solvation
is solvation parameter of atom i, i.e. a contribution to the free energy of solvation
of the particular atom i per surface unit area. The required solvation parameters for different types of atoms (C
, N
, O
, S
, etc.) are usually determined by least squares
fit of the calculated and experimental transfer free energies for a series of organic compound
s. The experimental energies are determined from partition coefficient
s of these compounds between different solutions or media using standard mole concentrations of the solutes.
It is noteworthy that solvation energy is free energy required to transfer a solute molecule from a solvent to “vacuum” (gas phase). This solvation energy can supplement the intramolecular energy in vacuum calculated in molecular mechanics
. Therefore, the required atomic solvation parameters were initially derived from water-gas partition data. However, the dielectric properties of proteins and lipid bilayer
s are much more similar to those of nonpolar solvents than to vacuum. Newer parameters have therefore been derived from water
-octanol
partition coefficient
s or other similar data. Such parameters actually describe transfer energy between two condensed media or the difference of two solvation energies.
(PB) describes the electrostatic environment of a solute in a solvent containing ion
s. It can be written in cgs units as: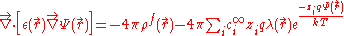
or (in mks):
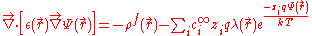
where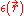 represents the position-dependent dielectric,
represents the position-dependent dielectric, 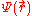 represents the electrostatic potential,
represents the electrostatic potential, 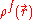 represents the charge density of the solute,
represents the charge density of the solute,  represents the concentration of the ion i at a distance of infinity from the solute,
represents the concentration of the ion i at a distance of infinity from the solute, 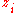 is the valence of the ion, q is the charge of a proton, k is the Boltzmann constant, T is the temperature
is the valence of the ion, q is the charge of a proton, k is the Boltzmann constant, T is the temperature
, and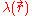 is a factor for the position-dependent accessibility of position r to the ions in solution (often set to uniformly 1). If the potential is not large, the equation can be linearized
is a factor for the position-dependent accessibility of position r to the ions in solution (often set to uniformly 1). If the potential is not large, the equation can be linearized
to be solved more efficiently.
A number of numerical Poisson-Boltzmann equation solvers of varying generality and efficiency have been developed, including one application with a specialized computer hardware platform. However, performance from PB solvers does not yet equal that from the more commonly used generalized Born approximation.
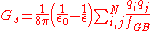
where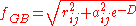
and

where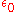 is the permittivity of free space,
is the permittivity of free space,  is the dielectric constant
is the dielectric constant
of the solvent being modeled,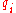 is the electrostatic charge on particle i,
is the electrostatic charge on particle i, 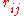 is the distance between particles i and j, and
is the distance between particles i and j, and  is a quantity (with the dimension of length) known as the effective Born radius. The effective Born radius of an atom characterizes its degree of burial inside the solute; qualitatively it can be thought of as the distance from the atom to the molecular surface. Accurate estimation of the effective Born radii is critical for the GB model.
is a quantity (with the dimension of length) known as the effective Born radius. The effective Born radius of an atom characterizes its degree of burial inside the solute; qualitatively it can be thought of as the distance from the atom to the molecular surface. Accurate estimation of the effective Born radii is critical for the GB model.
is known as MM/GBSA. Although this formulation has been shown to successfully identify the native state
s of short peptides with well-defined tertiary structure
, the conformational ensembles produced by GBSA models in other studies differ significantly from those produced by explicit solvent and do not identify the protein's native state. In particular, salt bridge
s are overstabilized, possibly due to insufficient electrostatic screening, and a higher-than-native alpha helix
population was observed. Variants of the GB model have also been developed to approximate the electrostatic environment of membranes, which have had some success in folding the transmembrane helices
of integral membrane protein
s.
Another strategy is implemented for the CHARMM
19 force-field and is called EEF1. EEF1 is based on a Gaussian-shaped solvent exclusion. The solvation free energy is
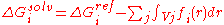
The reference solvation free energy of i corresponds to a suitably chosen small molecule in
which group i is essentially fully solvent-exposed. The integral is over the volume Vj of
group j and the summation is over all groups j around i. EEF1 additionally utilizes a distance-dependent (non-constant) dielectric, and ionic side-chains of proteins are simply neutralized. It is only 50% slower than a vacuum simulation. This model was later augmented with the hydrophobic effect and called Charmm19/SASA.
(PME) method of electrostatic calculation.
effects arising from solute-imposed constraints on the organization of the water or solvent molecules. This is known as the hydrophobic effect
and is a major factor in the folding
process of globular proteins with hydrophobic cores. Implicit solvation models may be augmented with a term that accounts for the hydrophobic effect. The most popular way to do this is by taking the solvent accessible surface area (SASA) as a proxy
of the extent of the hydrophobic effect. Most authors place the extent of this effect between 5 and 45 cal/(Å2 mol). Note that this surface area pertains to the solute, while the hydrophobic effect is mostly entropic in nature at physiological temperatures and occurs on the side of the solvent.
much faster. This acceleration means that more configurations are visited per simulated nanosecond, on top of whatever CPU acceleration is achieved in comparison to explicit solvent. But it can lead to misleading results when kinetics are of interest.
Viscosity may be added back by using Langevin dynamics
instead of Hamiltonian dynamics
and choosing an appropriate damping constant for the particular solvent. One should keep in mind, though, that the folding rate of proteins does not depend linearly on viscosity for all regimes.
s in the first solvation shell
are important for solubility of organic molecules and especially ions. Their average energetic contribution can be reproduced with an implicit solvent model.
tend to cluster together or occupy nonpolar media, whereas polar and charged groups of the solute tend to remain in water. However, it is important to properly balance the opposite energy contributions from different types of atoms. Several important points have been discussed and investigated over the years.
solution is a poor approximation of proteins or biological membranes because it contains ~2M of water, and that cyclohexane
would be a much better approximation. Investigation of passive permeability barriers for different compounds across lipid bilayers led to conclusion that 1,9-decadiene can serve as a good approximations of the bilayer interior, whereas octanol
was a very poor approximation. A set of solvation parameters derived for protein interior from protein engineering
data was also different from octanol
scale: it was close to cyclohexane
scale for nonpolar atoms but intermediate between cyclohexane and octanol scales for polar atoms. Thus, different atomic solvation parameters should be applied for modeling of protein folding and protein-membrane binding. This issue remains controversial. The original idea of the method was to derive all solvation parameters directly from experimental partition coefficient
s of organic molecules, which allows calculation of solvation free energy. However, some of the recently developed electrostatic models use ad hoc values of 20 or 40 cal/(Å2 mol) for all types of atoms. The non-existent “hydrophobic” interactions of polar atoms are overridden by large electrostatic energy penalties in such models.
or uniform media. It is possible to express van der Waals interaction energies in the solid
state in the surface energy units. This was sometimes done for interpreting protein engineering
and ligand binding energetics, which leads to “solvation” parameter for aliphatic carbon of ~40 cal/(Å2 mol), which is 2 times bigger than ~20 cal/(Å2 mol) obtained for transfer from water to liquid hydrocarbons, because the parameters derived by such fitting represent sum of the hydrophobic energy (i.e. 20 cal/Å2 mol) and energy of van der Waals attractions of aliphatic groups in the solid state, which corresponds to fusion enthalpy of alkanes. Unfortunately, the simplified ASA-based model can not capture the "specific" distance-dependent interactions between different types of atoms in the solid state which are responsible for clustering of atoms with similar polarities in protein structures and molecular crystals. Parameters of such interatomic interactions, together with atomic solvation parameters for the protein interior, have been approximately derived from protein engineering
data. The implicit solvation model breaks down when solvent molecules associate strongly with binding cavities in a protein, so that the protein and the solvent molecules form a continuous solid body. On the other hand, this model can be successfully applied for describing transfer from water to the fluid
lipid bilayer.
. This method was rarely tested for hundreds of protein structures.
and molecular dynamics
. The transfer of an ion from water to a nonpolar media with dielectric constant
of ~3 (lipid bilayer) or 4 to 10 (interior of proteins) costs significant energy, as follows from Born
equation and experiments. However, since the charged protein residues are ionizable, they simply lose their charges in the nonpolar environment, which costs relatively little at the neutral pH
: ~4 to 7 kcal/mol for Asp, Glu, Lys, and Arg amino acid
residues, according to the Henderson-Hasselbalch equation
, ΔG=2.3RT(pH-pK). The low energetic costs of such ionization effects have indeed been observed for protein mutants with buried ionizable residues. and hydrophobic α-helical peptides in membranes with a single ionizable residue in the middle. However, all electrostatic methods, such as PB, GB, or GBSA assume that ionizable groups remain charged in the nonpolar environments, which leads to grossly overestimated electrostatic energy. In the simplest accessible surface area
-based models, this problem was treated using different solvation parameters for charged atoms or Henderson-Hasselbalch equation with some modifications. However even the latter approach does not solve the problem. Charged residues can remain charged even in the nonpolar environment if they are involved in intramolecular ion pairs and H-bonds. Thus, the energetic penalties can be overestimated even using the Henderson-Hasselbalch equation. More rigorous theoretical methods describing such ionization effects have been developed, and there are ongoing efforts to incorporate such methods into the implicit solvation models.
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
as a continuous medium instead of individual “explicit” solvent molecules most often used in molecular dynamics
Molecular dynamics
Molecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
simulations and in other applications of molecular mechanics
Molecular mechanics
Molecular mechanics uses Newtonian mechanics to model molecular systems. The potential energy of all systems in molecular mechanics is calculated using force fields...
. The method is often applied to estimate free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
-solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
interactions in structural and chemical processes, such as folding or conformational transitions
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
of proteins, DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
, RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
, and polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s, association of biological macromolecules with ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s, or transport of drugs
DRUGS
Destroy Rebuild Until God Shows are an American post-hardcore band formed in 2010. They released their debut self-titled album on February 22, 2011.- Formation :...
across biological membrane
Biological membrane
A biological membrane or biomembrane is an enclosing or separatingmembrane that acts as a selective barrier, within or around a cell. It consists of a lipid bilayer with embedded proteins that may constitute close to 50% of membrane content...
s.
The implicit solvation model is justified in liquids, where the potential of mean force
Potential of mean force
The Potential of Mean Force of a system with N molecules is strictly the potential that gives the average force over all the configurations of all the n+1...N molecules acting on a particle at any fixed configuration keeping fixed a set of molecules 1...n...
can be applied to approximate the averaged behavior of many highly dynamic solvent molecules. However, the interiors of biological membrane
Biological membrane
A biological membrane or biomembrane is an enclosing or separatingmembrane that acts as a selective barrier, within or around a cell. It consists of a lipid bilayer with embedded proteins that may constitute close to 50% of membrane content...
s or protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s can also be considered as media with specific solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
or dielectric
Dielectric
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric is placed in an electric field, electric charges do not flow through the material, as in a conductor, but only slightly shift from their average equilibrium positions causing dielectric...
properties. These media are continuous but not necessarily uniform, since their properties can be described by different analytical functions, such as “polarity profiles” of lipid bilayer
Lipid bilayer
The lipid bilayer is a thin membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around cells. The cell membrane of almost all living organisms and many viruses are made of a lipid bilayer, as are the membranes surrounding the cell nucleus...
s. There are two basic types of implicit solvent methods: models based on accessible surface area
Accessible surface area
The accessible surface area is the surface area of a biomolecule that is accessible to a solvent. The ASA is usually quoted in square ångstrom . ASA was first described by Lee & Richards in 1971 and is sometimes called the Lee-Richards molecular surface...
s (ASA) that were historically the first, and more recent continuum electrostatics models, although various modifications and combinations of the different methods are possible.
The accessible surface area
Accessible surface area
The accessible surface area is the surface area of a biomolecule that is accessible to a solvent. The ASA is usually quoted in square ångstrom . ASA was first described by Lee & Richards in 1971 and is sometimes called the Lee-Richards molecular surface...
(ASA) method is based on experimental linear relations between Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of transfer and the surface area
Surface area
Surface area is the measure of how much exposed area a solid object has, expressed in square units. Mathematical description of the surface area is considerably more involved than the definition of arc length of a curve. For polyhedra the surface area is the sum of the areas of its faces...
of a solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
molecule. This method operates directly with free energy of solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
, unlike molecular mechanics
Molecular mechanics
Molecular mechanics uses Newtonian mechanics to model molecular systems. The potential energy of all systems in molecular mechanics is calculated using force fields...
or electrostatic methods that include only the enthalpic
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
component of free energy. The continuum representation of solvent also significantly improves the computational speed and reduces errors in statistical averaging that arise from incomplete sampling of solvent conformations, so that the energy landscapes obtained with implicit and explicit solvent are different. Although the implicit solvent model is useful for simulations of biomolecules, this is an approximate method with certain limitations and problems related to parameterization and treatment of ionization
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
effects.
Accessible surface area-based method
The free energy of solvation of a soluteSolution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
molecule in the simplest ASA-based method is given by:
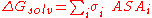
where
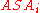 is accessible surface area
is accessible surface areaAccessible surface area
The accessible surface area is the surface area of a biomolecule that is accessible to a solvent. The ASA is usually quoted in square ångstrom . ASA was first described by Lee & Richards in 1971 and is sometimes called the Lee-Richards molecular surface...
of atom i, and
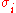 is solvation parameter of atom i, i.e. a contribution to the free energy of solvation
is solvation parameter of atom i, i.e. a contribution to the free energy of solvationSolvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
of the particular atom i per surface unit area. The required solvation parameters for different types of atoms (C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, S
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, etc.) are usually determined by least squares
Least squares
The method of least squares is a standard approach to the approximate solution of overdetermined systems, i.e., sets of equations in which there are more equations than unknowns. "Least squares" means that the overall solution minimizes the sum of the squares of the errors made in solving every...
fit of the calculated and experimental transfer free energies for a series of organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s. The experimental energies are determined from partition coefficient
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
s of these compounds between different solutions or media using standard mole concentrations of the solutes.
It is noteworthy that solvation energy is free energy required to transfer a solute molecule from a solvent to “vacuum” (gas phase). This solvation energy can supplement the intramolecular energy in vacuum calculated in molecular mechanics
Molecular mechanics
Molecular mechanics uses Newtonian mechanics to model molecular systems. The potential energy of all systems in molecular mechanics is calculated using force fields...
. Therefore, the required atomic solvation parameters were initially derived from water-gas partition data. However, the dielectric properties of proteins and lipid bilayer
Lipid bilayer
The lipid bilayer is a thin membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around cells. The cell membrane of almost all living organisms and many viruses are made of a lipid bilayer, as are the membranes surrounding the cell nucleus...
s are much more similar to those of nonpolar solvents than to vacuum. Newer parameters have therefore been derived from water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
-octanol
Octanol
Octanol is a straight chain fatty alcohol with eight carbon atoms and the molecular formula CH37OH. Although the term octanol usually refers exclusively to the primary alcohol 1-octanol, there are other less common isomers of octanol such as the secondary alcohols 2-octanol, 3-octanol and...
partition coefficient
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
s or other similar data. Such parameters actually describe transfer energy between two condensed media or the difference of two solvation energies.
Poisson-Boltzmann
Although this equation has solid theoretical justification, it is computationally expensive to calculate without approximations. The Poisson-Boltzmann equationPoisson-Boltzmann equation
The Poisson–Boltzmann equation is a differential equation that describes electrostatic interactions between molecules in ionic solutions. It is the mathematical base for the Gouy–Chapman double layer theory; first proposed by Gouy in 1910 and complemented by Chapman in 1913...
(PB) describes the electrostatic environment of a solute in a solvent containing ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s. It can be written in cgs units as:
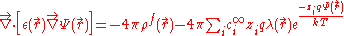
or (in mks):
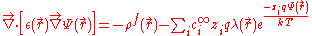
where
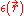 represents the position-dependent dielectric,
represents the position-dependent dielectric, 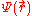 represents the electrostatic potential,
represents the electrostatic potential, 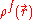 represents the charge density of the solute,
represents the charge density of the solute,  represents the concentration of the ion i at a distance of infinity from the solute,
represents the concentration of the ion i at a distance of infinity from the solute, 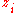 is the valence of the ion, q is the charge of a proton, k is the Boltzmann constant, T is the temperature
is the valence of the ion, q is the charge of a proton, k is the Boltzmann constant, T is the temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, and
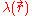 is a factor for the position-dependent accessibility of position r to the ions in solution (often set to uniformly 1). If the potential is not large, the equation can be linearized
is a factor for the position-dependent accessibility of position r to the ions in solution (often set to uniformly 1). If the potential is not large, the equation can be linearizedLinearization
In mathematics and its applications, linearization refers to finding the linear approximation to a function at a given point. In the study of dynamical systems, linearization is a method for assessing the local stability of an equilibrium point of a system of nonlinear differential equations or...
to be solved more efficiently.
A number of numerical Poisson-Boltzmann equation solvers of varying generality and efficiency have been developed, including one application with a specialized computer hardware platform. However, performance from PB solvers does not yet equal that from the more commonly used generalized Born approximation.
Generalized Born
The Generalized Born (GB) model is an approximation to the exact (linearized) Poisson-Boltzmann equation. It is based on modeling the protein as a set of spheres whose internal dielectric constant differs from the external solvent. The model has the following functional form: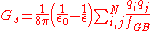
where
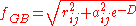
and

where
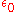 is the permittivity of free space,
is the permittivity of free space,  is the dielectric constant
is the dielectric constantDielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
of the solvent being modeled,
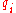 is the electrostatic charge on particle i,
is the electrostatic charge on particle i, 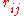 is the distance between particles i and j, and
is the distance between particles i and j, and  is a quantity (with the dimension of length) known as the effective Born radius. The effective Born radius of an atom characterizes its degree of burial inside the solute; qualitatively it can be thought of as the distance from the atom to the molecular surface. Accurate estimation of the effective Born radii is critical for the GB model.
is a quantity (with the dimension of length) known as the effective Born radius. The effective Born radius of an atom characterizes its degree of burial inside the solute; qualitatively it can be thought of as the distance from the atom to the molecular surface. Accurate estimation of the effective Born radii is critical for the GB model.GBSA
GBSA is simply a Generalized Born model augmented with the hydrophobic solvent accessible surface area SA term. It is among the most commonly used implicit solvent model combinations. The use of this model in the context of molecular mechanicsMolecular mechanics
Molecular mechanics uses Newtonian mechanics to model molecular systems. The potential energy of all systems in molecular mechanics is calculated using force fields...
is known as MM/GBSA. Although this formulation has been shown to successfully identify the native state
Native state
In biochemistry, the native state of a protein is its operative or functional form. While all protein molecules begin as simple unbranched chains of amino acids, once completed they assume highly specific three-dimensional shapes; that ultimate shape, known as tertiary structure, is the folded...
s of short peptides with well-defined tertiary structure
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
, the conformational ensembles produced by GBSA models in other studies differ significantly from those produced by explicit solvent and do not identify the protein's native state. In particular, salt bridge
Salt bridge (protein)
Salt bridges fall into the broader category of noncovalent interactions. A salt bridge is actually a combination of two noncovalent interactions: hydrogen bonding and electrostatic interactions . This is most commonly observed to contribute stability to the entropically unfavorable folded...
s are overstabilized, possibly due to insufficient electrostatic screening, and a higher-than-native alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
population was observed. Variants of the GB model have also been developed to approximate the electrostatic environment of membranes, which have had some success in folding the transmembrane helices
Transmembrane helix
Transmembrane domain usually denotes a single transmembrane alpha helix of a transmembrane protein. It is called a "domain" because an alpha-helix in a membrane can fold independently from the rest of the protein, similar to domains of water-soluble proteins...
of integral membrane protein
Integral membrane protein
An integral membrane protein is a protein molecule that is permanently attached to the biological membrane. Proteins that cross the membrane are surrounded by "annular" lipids, which are defined as lipids that are in direct contact with a membrane protein...
s.
Ad-hoc fast solvation models
Another possibility is to use ad-hoc quick strategies to estimate solvation free energy. A first generation of fast implicit solvents is based on the calculation of a per-atom solvent accessible surface area. For each of group of atom types, a different parameter scales its contribution to solvation ("ASA-based model" described above).Another strategy is implemented for the CHARMM
CHARMM
CHARMM is the name of a widely used set of force fields for molecular dynamics as well as the name for the molecular dynamics simulation and analysis package associated with them...
19 force-field and is called EEF1. EEF1 is based on a Gaussian-shaped solvent exclusion. The solvation free energy is
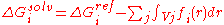
The reference solvation free energy of i corresponds to a suitably chosen small molecule in
which group i is essentially fully solvent-exposed. The integral is over the volume Vj of
group j and the summation is over all groups j around i. EEF1 additionally utilizes a distance-dependent (non-constant) dielectric, and ionic side-chains of proteins are simply neutralized. It is only 50% slower than a vacuum simulation. This model was later augmented with the hydrophobic effect and called Charmm19/SASA.
Hybrid implicit/explicit solvation models
It is possible to include a layer or sphere of water molecules around the solute, and model the bulk with an implicit solvent. Such an approach is proposed by M.S. Lee and coworkers. The bulk solvent is modeled with a Generalized Born approach and the multi-grid method used for Coulombic pairwise particle interactions. It is reported to be faster than a full explicit solvent simulation with the particle mesh EwaldEwald summation
Ewald summation, named after Paul Peter Ewald, is a method for computing the interaction energies of periodic systems , particularly electrostatic energies. Ewald summation is a special case of the Poisson summation formula, replacing the summation of interaction energies in real space with an...
(PME) method of electrostatic calculation.
The hydrophobic effect
Models like PB and GB allow estimation of the mean electrostatic free energy but do not account for the (mostly) entropicEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
effects arising from solute-imposed constraints on the organization of the water or solvent molecules. This is known as the hydrophobic effect
Hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in aqueous solution and exclude water molecules. The name, literally meaning "water-fearing," describes the segregation and apparent repulsion between water and nonpolar substances...
and is a major factor in the folding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
process of globular proteins with hydrophobic cores. Implicit solvation models may be augmented with a term that accounts for the hydrophobic effect. The most popular way to do this is by taking the solvent accessible surface area (SASA) as a proxy
Proxy (statistics)
In statistics, a proxy variable is something that is probably not in itself of any great interest, but from which a variable of interest can be obtained...
of the extent of the hydrophobic effect. Most authors place the extent of this effect between 5 and 45 cal/(Å2 mol). Note that this surface area pertains to the solute, while the hydrophobic effect is mostly entropic in nature at physiological temperatures and occurs on the side of the solvent.
Viscosity
Implicit solvent models such as PB, GB, and SASA lack the viscosity that water molecules impart by randomly colliding and impeding the motion of solutes through their van der Waals repulsion. In many cases, this is desirable because it makes sampling of configurations and phase spacePhase space
In mathematics and physics, a phase space, introduced by Willard Gibbs in 1901, is a space in which all possible states of a system are represented, with each possible state of the system corresponding to one unique point in the phase space...
much faster. This acceleration means that more configurations are visited per simulated nanosecond, on top of whatever CPU acceleration is achieved in comparison to explicit solvent. But it can lead to misleading results when kinetics are of interest.
Viscosity may be added back by using Langevin dynamics
Langevin dynamics
In physics, Langevin dynamics is an approach to the mathematical modeling of the dynamics of molecular systems, originally developed by the French physicist Paul Langevin...
instead of Hamiltonian dynamics
Hamiltonian mechanics
Hamiltonian mechanics is a reformulation of classical mechanics that was introduced in 1833 by Irish mathematician William Rowan Hamilton.It arose from Lagrangian mechanics, a previous reformulation of classical mechanics introduced by Joseph Louis Lagrange in 1788, but can be formulated without...
and choosing an appropriate damping constant for the particular solvent. One should keep in mind, though, that the folding rate of proteins does not depend linearly on viscosity for all regimes.
Hydrogen-bonds with solvent
Solute-solvent hydrogen bondHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s in the first solvation shell
Solvation shell
A Solvation shell is a shell of any chemical species acting as a solvent, surrounding a solute species. When the solvent is water it is often referred to as a hydration shell or hydration sphere....
are important for solubility of organic molecules and especially ions. Their average energetic contribution can be reproduced with an implicit solvent model.
Problems and limitations
All implicit solvation models rest on the simple idea that nonpolar atoms of a soluteSolution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
tend to cluster together or occupy nonpolar media, whereas polar and charged groups of the solute tend to remain in water. However, it is important to properly balance the opposite energy contributions from different types of atoms. Several important points have been discussed and investigated over the years.
Choice of model solvent
It has been noted that wet octanolOctanol
Octanol is a straight chain fatty alcohol with eight carbon atoms and the molecular formula CH37OH. Although the term octanol usually refers exclusively to the primary alcohol 1-octanol, there are other less common isomers of octanol such as the secondary alcohols 2-octanol, 3-octanol and...
solution is a poor approximation of proteins or biological membranes because it contains ~2M of water, and that cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
would be a much better approximation. Investigation of passive permeability barriers for different compounds across lipid bilayers led to conclusion that 1,9-decadiene can serve as a good approximations of the bilayer interior, whereas octanol
Octanol
Octanol is a straight chain fatty alcohol with eight carbon atoms and the molecular formula CH37OH. Although the term octanol usually refers exclusively to the primary alcohol 1-octanol, there are other less common isomers of octanol such as the secondary alcohols 2-octanol, 3-octanol and...
was a very poor approximation. A set of solvation parameters derived for protein interior from protein engineering
Protein engineering
Protein engineering is the process of developing useful or valuable proteins. It is a young discipline, with much research taking place into the understanding of protein folding and recognition for protein design principles....
data was also different from octanol
Octanol
Octanol is a straight chain fatty alcohol with eight carbon atoms and the molecular formula CH37OH. Although the term octanol usually refers exclusively to the primary alcohol 1-octanol, there are other less common isomers of octanol such as the secondary alcohols 2-octanol, 3-octanol and...
scale: it was close to cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
scale for nonpolar atoms but intermediate between cyclohexane and octanol scales for polar atoms. Thus, different atomic solvation parameters should be applied for modeling of protein folding and protein-membrane binding. This issue remains controversial. The original idea of the method was to derive all solvation parameters directly from experimental partition coefficient
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
s of organic molecules, which allows calculation of solvation free energy. However, some of the recently developed electrostatic models use ad hoc values of 20 or 40 cal/(Å2 mol) for all types of atoms. The non-existent “hydrophobic” interactions of polar atoms are overridden by large electrostatic energy penalties in such models.
Solid-state applications
Strictly speaking, ASA-based models should only be applied to describe solvation, i.e. energetics of transfer between liquidLiquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or uniform media. It is possible to express van der Waals interaction energies in the solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
state in the surface energy units. This was sometimes done for interpreting protein engineering
Protein engineering
Protein engineering is the process of developing useful or valuable proteins. It is a young discipline, with much research taking place into the understanding of protein folding and recognition for protein design principles....
and ligand binding energetics, which leads to “solvation” parameter for aliphatic carbon of ~40 cal/(Å2 mol), which is 2 times bigger than ~20 cal/(Å2 mol) obtained for transfer from water to liquid hydrocarbons, because the parameters derived by such fitting represent sum of the hydrophobic energy (i.e. 20 cal/Å2 mol) and energy of van der Waals attractions of aliphatic groups in the solid state, which corresponds to fusion enthalpy of alkanes. Unfortunately, the simplified ASA-based model can not capture the "specific" distance-dependent interactions between different types of atoms in the solid state which are responsible for clustering of atoms with similar polarities in protein structures and molecular crystals. Parameters of such interatomic interactions, together with atomic solvation parameters for the protein interior, have been approximately derived from protein engineering
Protein engineering
Protein engineering is the process of developing useful or valuable proteins. It is a young discipline, with much research taking place into the understanding of protein folding and recognition for protein design principles....
data. The implicit solvation model breaks down when solvent molecules associate strongly with binding cavities in a protein, so that the protein and the solvent molecules form a continuous solid body. On the other hand, this model can be successfully applied for describing transfer from water to the fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
lipid bilayer.
Importance of extensive testing
More testing is needed to evaluate the performance of different implicit solvation models and parameter sets. They are often tested only for a small set of molecules with very simple structure, such as hydrophobic and amphiphilic α-helicesAlpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
. This method was rarely tested for hundreds of protein structures.
Treatment of ionization effects
Ionization of charged groups has been neglected in continuum electrostatic models of implicit solvation, as well as in standard molecular mechanicsMolecular mechanics
Molecular mechanics uses Newtonian mechanics to model molecular systems. The potential energy of all systems in molecular mechanics is calculated using force fields...
and molecular dynamics
Molecular dynamics
Molecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
. The transfer of an ion from water to a nonpolar media with dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
of ~3 (lipid bilayer) or 4 to 10 (interior of proteins) costs significant energy, as follows from Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
equation and experiments. However, since the charged protein residues are ionizable, they simply lose their charges in the nonpolar environment, which costs relatively little at the neutral pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
: ~4 to 7 kcal/mol for Asp, Glu, Lys, and Arg amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues, according to the Henderson-Hasselbalch equation
Henderson-Hasselbalch equation
In chemistry, the Henderson–Hasselbalch equation describes the derivation of pH as a measure of acidity in biological and chemical systems...
, ΔG=2.3RT(pH-pK). The low energetic costs of such ionization effects have indeed been observed for protein mutants with buried ionizable residues. and hydrophobic α-helical peptides in membranes with a single ionizable residue in the middle. However, all electrostatic methods, such as PB, GB, or GBSA assume that ionizable groups remain charged in the nonpolar environments, which leads to grossly overestimated electrostatic energy. In the simplest accessible surface area
Accessible surface area
The accessible surface area is the surface area of a biomolecule that is accessible to a solvent. The ASA is usually quoted in square ångstrom . ASA was first described by Lee & Richards in 1971 and is sometimes called the Lee-Richards molecular surface...
-based models, this problem was treated using different solvation parameters for charged atoms or Henderson-Hasselbalch equation with some modifications. However even the latter approach does not solve the problem. Charged residues can remain charged even in the nonpolar environment if they are involved in intramolecular ion pairs and H-bonds. Thus, the energetic penalties can be overestimated even using the Henderson-Hasselbalch equation. More rigorous theoretical methods describing such ionization effects have been developed, and there are ongoing efforts to incorporate such methods into the implicit solvation models.

