
Bingel reaction
Encyclopedia
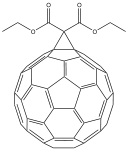
Fullerene chemistry
Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example fullerene is notoriously insoluble and adding a suitable group can enhance...
is a fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
cyclopropanation reaction
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
to a methanofullerene first discovered by C. Bingel in 1993 with the bromo
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
derivative of diethyl malonate
Diethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes...
in the presence of a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
such as sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
or DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
. The preferred double bonds for this reaction on the fullerene surface are the shorter bonds at the junctions of two hexagons (6-6 bonds) and the driving force is relief of steric strain.
The reaction is of importance in the field of chemistry because it allows the introduction of useful extensions to the fullerene sphere. These extensions alter their properties for instance solubility and electrochemical behavior and therefore widen the range of potential technical applications.
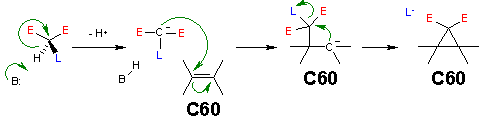
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for this reaction is as follows: a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
abstracts the acidic malonate proton generating a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
or enolate which reacts with the electron deficient fullerene double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
in a nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
. This in turn generates a carbanion which displaces bromine in a nucleophilic aliphatic substitution in an intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
ring cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
ring closure.
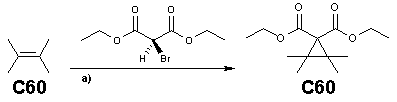
Scope
The Bingel reaction is a popular method in fullerene chemistry. The malonateMalonate
The malonate or propanedioate ion is CH222− . Malonate compounds include salts and esters of malonic acid, such as*diethyl malonate, 2,*dimethyl malonate, 2,...
(functionalized with the halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
atom) is often obtained in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
in a mixture of base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
and tetrachloromethane or iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
. The reaction is also known to take place with the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
groups replaced by alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
groups in dialkynylmethanofullerenes.
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
reaction. N-(Diphenylmethylene)glycinate Esters in a Bingel reaction take a different conjugate course and react to a fullerene dihydropyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
.

Retro-Bingel reaction
Protocols exist for the removal of the methano group based, on electrolytic reduction or amalgamated magnesiumMagnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...

