Perkow reaction
Encyclopedia
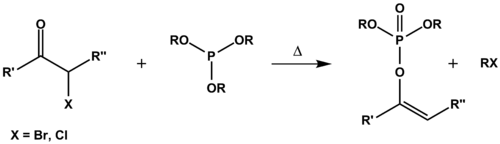
The Perkow reaction is an organic reaction
in which a trialkyl phosphite ester
reacts with a haloketone
to form a dialkyl vinyl
phosphate
and an alkyl halide.
 In the related Michaelis–Arbuzov reaction the same reactants are known to form a beta-keto phosphonate which is an important reagent
In the related Michaelis–Arbuzov reaction the same reactants are known to form a beta-keto phosphonate which is an important reagent
in the Horner-Wadsworth-Emmons reaction
on the road to alkene
s. The Perkow reaction, in this respect is considered a side-reaction.
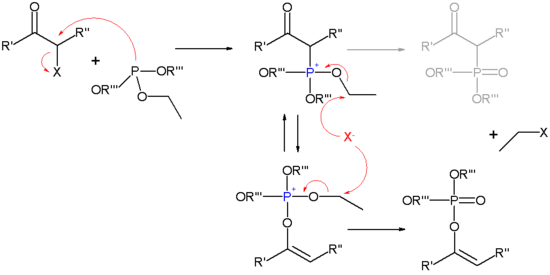
of the Perkow reaction consists of a nucleophilic displacement of the α-halogen atom by the phosphorus
nucleophile
. The phosphite ester salt is subject to keto-enol tautomerism
and if the enol
isomer is predominant the Perkow adduct is formed otherwise the keto form results in the Michaelis-Arbuzov adduct. The second step of the reaction is a second nucleophilic displacement of the halide anion on one of the phosphite alkoxide
substituent
s forming an enol phosphonium oxide.
and triethylphosphite which is able to engage in a secondary [4+3] cycloaddition
with furan
through the action of the base
sodium 2,2,2-trifluoroethoxide. The authors report mediocre yields.
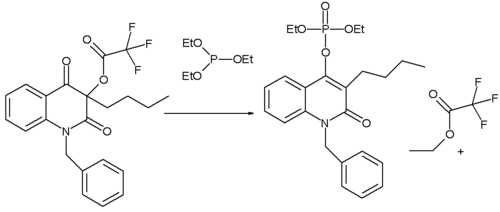
 The Perkow reaction is also used in the synthesis of novel quinoline
The Perkow reaction is also used in the synthesis of novel quinoline
s. When the substituent is n-butyl
the reaction product is the classical Perkow adduct. In this reaction the leaving group
is an electron deficient acyl group (owing to the presence of three fluorine groups). When the substituent on the other hand is phenyl (not shown) the phospite has a preference for reaction with the acyl group leading to an ethyl enol ether
. Key in explaining the difference in reactivity is the electron density on the α-keto carbon atom.
 Aryl enol
Aryl enol
phosphates formed in good yields (ca. 90%) in the Perkow reaction can be used as phosphorylating
reagents, e.g. able to transform AMP
into ATP
.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which a trialkyl phosphite ester
Phosphite ester
A phosphite ester or organophosphite is a type of chemical compound with the general structure P3. Phosphite esters can be considered as esters of phosphorous acid, H3PO3. A simple phosphite ester is trimethylphosphite, P3...
reacts with a haloketone
Haloketone
A haloketone in organic chemistry is a functional group consisting of a ketone group or more general a carbonyl group with a α-halogen substituent. The general structure is RR'CCR where R is an alkyl or aryl residue and X any one of the halogens...
to form a dialkyl vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
and an alkyl halide.

Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
in the Horner-Wadsworth-Emmons reaction
Horner-Wadsworth-Emmons reaction
The Horner-Wadsworth-Emmons reaction is the chemical reaction of stabilized phosphonate carbanions with aldehydes to produce predominantly E-alkenes....
on the road to alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s. The Perkow reaction, in this respect is considered a side-reaction.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of the Perkow reaction consists of a nucleophilic displacement of the α-halogen atom by the phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. The phosphite ester salt is subject to keto-enol tautomerism
Keto-enol tautomerism
In organic chemistry, keto-enol tautomerism refers to a chemical equilibrium between a keto form and an enol . The enol and keto forms are said to be tautomers of each other...
and if the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
isomer is predominant the Perkow adduct is formed otherwise the keto form results in the Michaelis-Arbuzov adduct. The second step of the reaction is a second nucleophilic displacement of the halide anion on one of the phosphite alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s forming an enol phosphonium oxide.

Scope
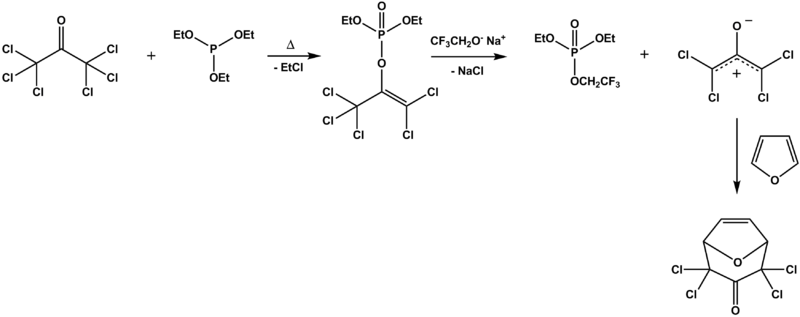
The Perkow reaction has been applied in the synthesis of a novel insect repellent based on hexachloroacetoneHexachloroacetone
Hexachloroacetone is an organic compound with the formula Cl3C-CO-CCl3. It is also called hexachloropropanone or perchloroacetone. Numbers indicating the position of the chlorine-atoms are generally omitted as all the possible positions are substituted with chlorine. It is a colorless liquid,...
and triethylphosphite which is able to engage in a secondary [4+3] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
with furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
through the action of the base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
sodium 2,2,2-trifluoroethoxide. The authors report mediocre yields.

Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
s. When the substituent is n-butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
the reaction product is the classical Perkow adduct. In this reaction the leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
is an electron deficient acyl group (owing to the presence of three fluorine groups). When the substituent on the other hand is phenyl (not shown) the phospite has a preference for reaction with the acyl group leading to an ethyl enol ether
Enol ether
An enol ether is an alkene with an alkoxy substituent. The general structure is R_1R_2C=CR_3-O-R_4 with R an alkyl or an aryl group. Enol ethers and enamines are so-called activated alkenes or electron rich alkenes because the oxygen atom donates electrons to the double bond by forming a resonance...
. Key in explaining the difference in reactivity is the electron density on the α-keto carbon atom.

Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
phosphates formed in good yields (ca. 90%) in the Perkow reaction can be used as phosphorylating
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
reagents, e.g. able to transform AMP
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
into ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
.

