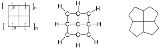
Fenestrane
Encyclopedia
A fenestrane in organic chemistry
is a type of chemical compound
with a central quaternary carbon atom which serves as a common vertex for four fused carbocycles. They can be regarded as spiro compound
s twice over. Because of their inherent strain
and instability fenestranes are of theoretical interest to chemists. The name proposed in 1972 by Vlasios Georgian and Martin Saltzman is derived from the Latin
word for window
: Fenestra.
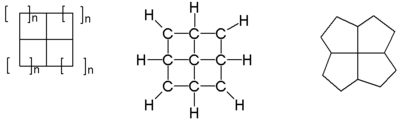 The smallest member of the family consisting of 4 fused cyclopropane
The smallest member of the family consisting of 4 fused cyclopropane
rings is [3,3,3,3]fenestrane or pyramidane a molecule related to tetrahedrane
with an extensive history on its own. In the next member 4 cyclobutane
rings are fused to the archetypical window motif. It is called in its own chemical nomenclature [4,4,4,4]fenestrane simply by counting the number of carbon atoms in each ring. The formal name for this compound is less catchy: tetracyclo[3.3.1.03,9.07,9]nonane.
In an extreme case the central carbon atom ordinarily with a tetrahedral molecular geometry
gets completely flattened. In the molecular orbital
picture for square planar methane
two of a total of 3 sp2 hybridized carbon atomic orbitals form regular bonds with two of the hydrogen atoms as in a planar alkene
. The third sp2 orbital interacts in a three-center two-electron bond
with the two remaining hydrogen atoms utilizing only the hydrogen electrons. Two additional carbon valence electron
s are situated in a p-orbital perpendicular to the plane of the molecule. The four C-H bonds are equal because they resonate. In silico
calculations show that it takes 95 to 250 kcal/mol (400 to 1,050 kJ/mol) for this process.
One of the highest strained fenestranes actually isolated is a [4,4,4,5]fenestrane with bond angles through the central carbon atom of around 130° based on X-ray diffraction. In this molecule the bonds extending from the central carbon atom are shortened with bond length
s of 149 picometer while those at the perimeter are extended to 159 pm. (The C-C bond in ethane
is 155 pm long.)
The first ever synthesized fenestrane is a [4,5,5,6]fenestrane:
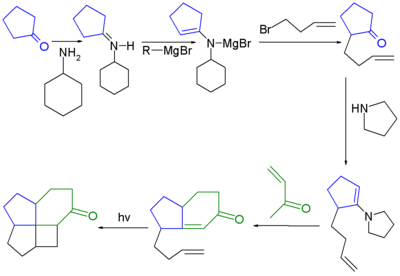
compounds and their salts are more likely to form crystalline compounds suitable for x-ray analysis. In step 1 the alkyl halide 1-iodo-3-butene 1 is converted to a cyanozinc cuprate
2 (by transmetalation
of the organozinc iodide with copper cyanide) which reacts in the next step with 1-nitro-cyclopentene 3 in a nucleophilic addition
whereby the nitronate
4 is captured by phenylselenenyl bromide to the selenium intermediate 5. Hydrogen peroxide
oxidation of 5 yields the nitroalkene 6 as a mixture of syn and anti isomers. A [4+2]cycloaddition
with n-butyl-enol ether
in presence of trimethylaluminium
gives the nitronate
7 and a second [3+2]cycloaddition by heating in presence of potassium carbonate
gives the nitroso
acetal
8. Hydrogenation
with Raney nickel
gives the diol
9 which on a double Mitsunobu reaction
(with an amine proton donor) gives the azafenestrane 10 as the borane
salt.
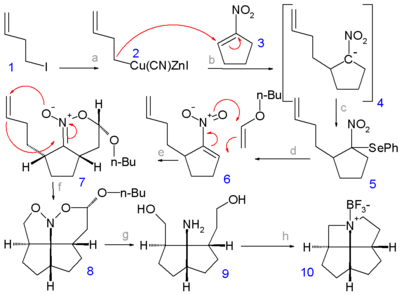 In the borane
In the borane
salt the N-C-C bond angle is 126°.
One study describes an unusual 8π disrotatory
- 6π conrotatory electrocyclic cascade reaction
aiming to minimise the number of steps required to synthesise a fenestrane.

Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a type of chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with a central quaternary carbon atom which serves as a common vertex for four fused carbocycles. They can be regarded as spiro compound
Spiro compound
A spiro compound is a bicyclic organic compound with rings connected through just one atom. The rings can be different in nature or identical. The connecting atom is also called the spiroatom, most often a quaternary carbon...
s twice over. Because of their inherent strain
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
and instability fenestranes are of theoretical interest to chemists. The name proposed in 1972 by Vlasios Georgian and Martin Saltzman is derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
word for window
Window
A window is a transparent or translucent opening in a wall or door that allows the passage of light and, if not closed or sealed, air and sound. Windows are usually glazed or covered in some other transparent or translucent material like float glass. Windows are held in place by frames, which...
: Fenestra.

Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
rings is [3,3,3,3]fenestrane or pyramidane a molecule related to tetrahedrane
Tetrahedrane
Tetrahedrane is a platonic hydrocarbon with chemical formula 44 and a tetrahedral structure. Extreme angle strain prevents this molecule from forming naturally....
with an extensive history on its own. In the next member 4 cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
rings are fused to the archetypical window motif. It is called in its own chemical nomenclature [4,4,4,4]fenestrane simply by counting the number of carbon atoms in each ring. The formal name for this compound is less catchy: tetracyclo[3.3.1.03,9.07,9]nonane.
In an extreme case the central carbon atom ordinarily with a tetrahedral molecular geometry
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
gets completely flattened. In the molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
picture for square planar methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
two of a total of 3 sp2 hybridized carbon atomic orbitals form regular bonds with two of the hydrogen atoms as in a planar alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. The third sp2 orbital interacts in a three-center two-electron bond
Three-center two-electron bond
A three-center two-electron bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons go into the bonding orbital, resulting in a...
with the two remaining hydrogen atoms utilizing only the hydrogen electrons. Two additional carbon valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s are situated in a p-orbital perpendicular to the plane of the molecule. The four C-H bonds are equal because they resonate. In silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
calculations show that it takes 95 to 250 kcal/mol (400 to 1,050 kJ/mol) for this process.
One of the highest strained fenestranes actually isolated is a [4,4,4,5]fenestrane with bond angles through the central carbon atom of around 130° based on X-ray diffraction. In this molecule the bonds extending from the central carbon atom are shortened with bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s of 149 picometer while those at the perimeter are extended to 159 pm. (The C-C bond in ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
is 155 pm long.)
The first ever synthesized fenestrane is a [4,5,5,6]fenestrane:

Current research
In a recent effort a [4,5,5,5]fenestrane was synthesized with one carbon atom replaced by nitrogen because aza-Aza-
The prefix aza- is used in organic chemistry to form names of organic compounds where a carbon atom is replaced by a nitrogen atom. Sometimes a number between hyphens is inserted before it to state which atom the nitrogen atom replaces...
compounds and their salts are more likely to form crystalline compounds suitable for x-ray analysis. In step 1 the alkyl halide 1-iodo-3-butene 1 is converted to a cyanozinc cuprate
Organocopper compound
Organocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions...
2 (by transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
of the organozinc iodide with copper cyanide) which reacts in the next step with 1-nitro-cyclopentene 3 in a nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
whereby the nitronate
Nitronate
A nitronate in organic chemistry is a functional group with the general structure R1R2C=N+2-).It is the anion of a nitronic acid, a tautomeric form of a nitro compound. A nitronic acid is also called a aci form...
4 is captured by phenylselenenyl bromide to the selenium intermediate 5. Hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
oxidation of 5 yields the nitroalkene 6 as a mixture of syn and anti isomers. A [4+2]cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
with n-butyl-enol ether
Enol ether
An enol ether is an alkene with an alkoxy substituent. The general structure is R_1R_2C=CR_3-O-R_4 with R an alkyl or an aryl group. Enol ethers and enamines are so-called activated alkenes or electron rich alkenes because the oxygen atom donates electrons to the double bond by forming a resonance...
in presence of trimethylaluminium
Trimethylaluminium
Trimethylaluminium is the chemical compound with the formula Al26, abbreviated as Al2Me6, 2 or the abbreviation TMA. This pyrophoric, colorless liquid is an industrially important organoaluminium compound...
gives the nitronate
Nitronate
A nitronate in organic chemistry is a functional group with the general structure R1R2C=N+2-).It is the anion of a nitronic acid, a tautomeric form of a nitro compound. A nitronic acid is also called a aci form...
7 and a second [3+2]cycloaddition by heating in presence of potassium carbonate
Potassium carbonate
Potassium carbonate is a white salt, soluble in water , which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid...
gives the nitroso
Nitroso
Nitroso refers to a functional group in organic chemistry which has the general formula RNO. Nitroso compounds are a class of organic compounds containing the nitroso functional group, R−N=O....
acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
8. Hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
with Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
gives the diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
9 which on a double Mitsunobu reaction
Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate or diisopropyl azodicarboxylate . The alcohol undergoes an inversion of stereochemistry...
(with an amine proton donor) gives the azafenestrane 10 as the borane
Borane
In chemistry, a borane is a chemical compound of boron and hydrogen. The boranes comprise a large group of compounds with the generic formulae of BxHy. These compounds do not occur in nature. Many of the boranes readily oxidise on contact with air, some violently. The parent member BH3 is called...
salt.

Borane
In chemistry, a borane is a chemical compound of boron and hydrogen. The boranes comprise a large group of compounds with the generic formulae of BxHy. These compounds do not occur in nature. Many of the boranes readily oxidise on contact with air, some violently. The parent member BH3 is called...
salt the N-C-C bond angle is 126°.
One study describes an unusual 8π disrotatory
Disrotatory
In a conrotatory mode of an electrocyclic reaction the substituents located at the termini of a conjugated double bond system move in the same direction during ring opening or ring closure...
- 6π conrotatory electrocyclic cascade reaction
Cascade reaction
A cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many...
aiming to minimise the number of steps required to synthesise a fenestrane.


