
Crystallographic defects in diamond
Encyclopedia

Crystallographic defect
Crystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
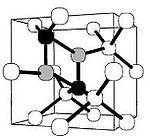
in the crystal lattice of diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
are common. Such crystallographic defects in diamond may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth. They affect the material properties of diamond
Material properties of diamond
Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. Diamond is an optically isotropic crystal that is transparent to opaque. Owing to its strong covalent bonding, diamond is the hardest naturally occurring material...
and determine to which type a diamond is assigned; the most dramatic effects are on the diamond color
Diamond color
A chemically pure and structurally perfect diamond is perfectly transparent with no hue, or color. However, in reality almost no gem-sized natural diamonds are absolutely perfect. The color of a diamond may be affected by chemical impurities and/or structural defects in the crystal lattice...
and electrical conductivity
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
, as explained by the band theory.
The defects can be detected by different types of spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, including electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
(EPR), luminescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
induced by light (photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
, PL) or electron beam (cathodoluminescence
Cathodoluminescence
Cathodoluminescence is an optical and electrical phenomenon whereby a beam of electrons is generated by an electron gun and then impacts on a luminescent material such as a phosphor, causing the material to emit visible light. The most common example is the screen of a television...
, CL), and absorption of light in the infrared (IR), visible and UV parts of the spectrum. Absorption spectrum is used not only to identify the defects, but also to estimate their concentration; it can also distinguish natural from synthetic
Synthetic diamond
Synthetic diamond is diamond produced in a technological process; as opposed to natural diamond, which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond, denoting the production method, High-Pressure High-Temperature synthesis and Chemical...
or enhanced diamonds
Diamond enhancement
Diamond enhancements are specific treatments, performed on natural diamonds , which are designed to improve the gemological characteristics — and therefore the value — of the stone in one or more ways...
.
The number of defects in diamond whose microscopic structure has been reliably identified is rather large (many dozens), and only the major ones are briefly mentioned in this article.
Labeling of diamond centers
There is a tradition in diamond spectroscopy to label a defect-induced spectrum by a numbered acronym (e.g. GR1). This tradition has been followed in general with some notable deviations, such as A, B and C centers. Many acronyms are confusing though:- Some symbols are too similar (e.g., 3H and H3).
- Accidentally, same labels were given to different centers detected by EPR and optical techniques (e.g., N3 EPR center and N3 optical center have no relation).
- Whereas some acronyms are logical, such as N3 (N for natural, i.e. observed in natural diamond) or H3 (H for heated, i.e. observed after irradiation and heating), many are not. In particular, there is no clear distinction between the meaning of labels GR (general radiation), R (radiation) and TR (type-II radiation).
Defect symmetry
The symmetry of defects in crystals is described by the point groupPoint group
In geometry, a point group is a group of geometric symmetries that keep at least one point fixed. Point groups can exist in a Euclidean space with any dimension, and every point group in dimension d is a subgroup of the orthogonal group O...
s. They differ from the space groups
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
describing the symmetry of crystals by absence of translations, and thus are much fewer in number. In diamond, only defects of the following symmetries have been observed thus far: tetrahedral (Td), tetragonal (D2d), trigonal (D3d,C3v), rhombic
Rhombus
In Euclidean geometry, a rhombus or rhomb is a convex quadrilateral whose four sides all have the same length. The rhombus is often called a diamond, after the diamonds suit in playing cards, or a lozenge, though the latter sometimes refers specifically to a rhombus with a 45° angle.Every...
(C2v), monoclinic (C2h, C1h, C2) and triclinic (C1 or CS).
The defect symmetry allows predicting many optical properties. For example, one-phonon (infrared) absorption in pure diamond lattice is forbidden because the lattice has an inversion center
Centrosymmetry
The term centrosymmetric, as generally used in crystallography, refers to a space group which contains an inversion center as one of its symmetry elements. In such a space group, for every point in the unit cell there is an indistinguishable point...
. However, introducing any defect (even "very symmetrical", such as N-N substitutional pair) breaks the crystal symmetry resulting in defect-induced infrared absorption, which is the most common tool to measure the defect concentrations in diamond.
An interesting symmetry related phenomenon has been observed that when diamond is produced by the high-pressure high-temperature synthesis, non-tetrahedral defects align to the direction of the growth. Such alignment has been also been observed in gallium arsenide and thus is not unique to diamond.
Extrinsic defects
Various elemental analyses of diamond reveal a wide range of impurities. They however mostly originate from inclusions of foreign materials in diamond, which could be nanometer-small and invisible in an optical microscopeOptical microscope
The optical microscope, often referred to as the "light microscope", is a type of microscope which uses visible light and a system of lenses to magnify images of small samples. Optical microscopes are the oldest design of microscope and were possibly designed in their present compound form in the...
. Also, virtually any element can be hammered into diamond by ion implantation
Ion implantation
Ion implantation is a materials engineering process by which ions of a material are accelerated in an electrical field and impacted into another solid. This process is used to change the physical, chemical, or electrical properties of the solid...
. More essential are elements which can be introduced into the diamond lattice as isolated atoms (or small atomic clusters) during the diamond growth. By 2008, those elements are nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
and perhaps sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. Manganese
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
and tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
have been unambiguously detected in diamond, but they might originate from foreign inclusions. Detection of isolated iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
in diamond has later been re-interpreted in terms of micro-particles of ruby
Ruby
A ruby is a pink to blood-red colored gemstone, a variety of the mineral corundum . The red color is caused mainly by the presence of the element chromium. Its name comes from ruber, Latin for red. Other varieties of gem-quality corundum are called sapphires...
produced during the diamond synthesis. Oxygen is believed to be a major impurity in diamond, but it has not been spectroscopically identified in diamond yet. Two electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
centers (OK1 and N3) have been assigned to nitrogen–oxygen complexes. However, the assignment is indirect and the corresponding concentrations are rather low (few parts per million).
Nitrogen
The most common impurity in diamond is nitrogen, which can comprise up to 1% of a diamond by mass. Previously, all lattice defects in diamond were thought to be the result of structural anomalies; later research revealed nitrogen to be present in most diamonds and in many different configurations. Most nitrogen enters the diamond lattice as a single atom (i.e. nitrogen-containing molecules dissociate before incorporation), however, molecular nitrogen incorporates into diamond as well.Absorption of light and other material properties of diamond are highly dependent upon nitrogen content and aggregation state. Although all aggregate configurations cause absorption in the infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
, diamonds containing aggregated nitrogen are usually colorless, i.e. have little absorption in the visible spectrum. The four main nitrogen forms are as follows:
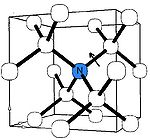
C-nitrogen center
The C center corresponds to electrically neutral single substitutional nitrogen atomAtom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s in the diamond lattice. These are easily seen in electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
spectra (in which they are confusingly called P1 centers). C centers impart a deep yellow to brown color; these diamonds are classed as type Ib and are commonly known as "canary diamonds", which are rare in gem
Gemstone
A gemstone or gem is a piece of mineral, which, in cut and polished form, is used to make jewelry or other adornments...
form. Most synthetic diamonds produced by high-pressure high-temperature (HPHT) technique contain a high level of nitrogen in the C form; nitrogen impurity originates from the atmosphere or from the graphite source. One nitrogen atom per 100,000 carbon atoms will produce yellow color. Because the nitrogen atoms have five available electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s (one more than the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms they replace), they act as "deep donor
Electron donor
An electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process....
s"; that is, each substituting nitrogen has an extra electron to donate and forms a donor energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
within the band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
. Light with energy above ~2.2 eV can excite the donor electrons into the conduction band
Conduction band
In the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
, resulting in the yellow color.
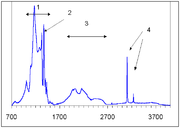
The C center produces a characteristic infrared absorption spectrum with a sharp peak at 1344 cm−1 and a broader feature at 1130 cm−1. Absorption at those peaks is routinely used to measure the concentration of single nitrogen. Another proposed way, using the UV absorption at ~260 nm, has later been discarded as unreliable.
Acceptor defects in diamond ionize the fifth nitrogen electron in the C center converting it into C+ center. The latter has a characteristic IR absorption spectrum with a sharp peak at 1332 cm−1 and broader and weaker peaks at 1115, 1046 and 950 cm−1.
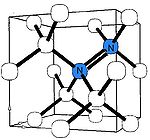
A-nitrogen center
The A center is probably the most common defect in natural diamonds. It consists of a neutral nearest-neighbor pair of nitrogen atoms substituting for the carbon atoms. The A center produces UV absorption threshold at ~4 eV (310 nm, i.e. invisible to eye) and thus causes no coloration. Diamond containing nitrogen predominantly in the A form as classed as type IaA.The A center is diamagnetic, but if ionized by UV light or deep acceptors, it produces an electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
spectrum W24, whose analysis unambiguously proves the N=N structure.
The A center shows an IR absorption spectrum with no sharp features, which is distinctly different from that of the C or B centers. Its strongest peak at 1282 cm−1 is routinely used to estimate the nitrogen concentration in the A form.
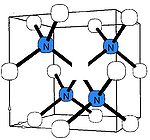
B-nitrogen center
There is a general consensus that B center (sometimes called B1) consists of a carbon vacancy surrounded by four nitrogen atoms substituting for carbon atoms. This model is consistent with other experimental results, but there is no direct spectroscopic data corroborating it. Diamonds where most nitrogen forms B centers are rare and are classed as type IaB; most gem diamonds contain a mixture of A and B centers, together with N3 centers.Similar to the A centers, B centers do not induce color, and no UV or visible absorption can be attributed to the B centers. Early assignment of the N9 absorption system to the B center have been disproven later. The B center has a characteristic IR absorption spectrum (see the infrared absorption picture above) with a sharp peak at 1332 cm−1 and a broader feature at 1280 cm−1. The latter is routinely used to estimate the nitrogen concentration in the B form.
Note that many optical peaks in diamond accidentally have similar spectral positions, which causes much confusion among gemologists. Spectroscopists use for defect identification the whole spectrum rather than one peak, and consider the history of the growth and processing of individual diamond.
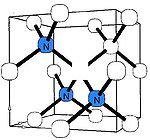
N3 nitrogen center
The N3 center consists of three nitrogen atoms surrounding a vacancy. Its concentration is always just a fraction of the A and B centers. The N3 center is paramagneticParamagnetism
Paramagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
, so its structure is well justified from the analysis of the EPR spectrum P2. This defect produces a characteristic absorption and luminescence line at 415 nm and thus does not induce color on its own. However, the N3 center is always accompanied by the N2 center, having an absorption line at 478 nm (and no luminescence). As a result, diamonds rich in N3/N2 centers are yellow in color.
Boron
Diamonds containing boron as a substitutional impurity are termed type IIb. Only one percent of natural diamonds are of this type, and most are blue to grey. Boron is acceptor in diamond: boron atoms have one less available electron than the carbon atoms; therefore, each boron atom substituting for a carbon atom creates an electron holeElectron hole
An electron hole is the conceptual and mathematical opposite of an electron, useful in the study of physics, chemistry, and electrical engineering. The concept describes the lack of an electron at a position where one could exist in an atom or atomic lattice...
in the band gap that can accept an electron from the valence band
Valence band
In solids, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature....
. This allows red light absorption, and due to the small energy (0.37 eV) needed for the electron to leave the valence band, holes can be thermally released from the boron atoms to the valence band
Valence band
In solids, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature....
even at room temperatures. These holes can move in an electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
and render the diamond electrically conductive (i.e., a p-type semiconductor
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
). Few boron atoms are required for this to happen—a typical ratio is one boron atom per 1,000,000 carbon atoms.
Boron-doped diamonds transmit light down to ~250 nm and absorb some red and infrared light (hence the blue color); they may phosphoresce
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
blue after exposure to shortwave ultraviolet light. Apart from optical absorption, boron acceptors have been detected by electron paramagnetic resonance.
Phosphorus
Phosphorus could be intentionally introduced into diamond grown by chemical vapor deposition (CVD) at concentrations up to ~0.01%. Phosphorus substitutes carbon in the diamond lattice. Similar to nitrogen, phosphorus has one more electron than carbon and thus acts as a donor; however, the ionization energy of phosphorus (0.6 eV) is much smaller than that of nitrogen (1.7 eV) and is small enough for room-temperature thermal ionization. This important property of phosphorus in diamond favors electronic applications, such as UV light emitting diodes (LEDLEd
LEd is a TeX/LaTeX editing software working under Microsoft Windows. It is a freeware product....
s, at 235 nm).
Hydrogen
Hydrogen is one of the most technological important impurities in semiconductors, including diamond. Hydrogen-related defects are very different in natural diamond and in synthetic diamond films. Those films are produced by various chemical vapor depositionChemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
(CVD) techniques in an atmosphere rich in hydrogen (typical hydrogen/carbon ratio >100), under strong bombardment of growing diamond by the plasma ions. As a result, CVD diamond is always rich in hydrogen and lattice vacancies. In polycrystalline films, much of the hydrogen may be located at the boundaries between diamond 'grains', or in non-diamond carbon inclusions. Within the diamond lattice itself, hydrogen-vacancy and hydrogen-nitrogen-vacancy complexes have been identified in negative charge states by electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
. In addition, numerous hydrogen-related IR absorption peaks are documented.
It is experimentally demonstrated that hydrogen passivates electrically active boron and phosphorus impurities. As a result of such passivation, shallow donor centers are presumably produced.
In natural diamonds, several hydrogen-related IR absorption peaks are commonly observed; the strongest ones are located at 1405, 3107 and 3237 cm−1 (see IR absorption figure above). The microscopic structure of the corresponding defects is yet unknown and it is not even certain whether or not those defects originate in diamond or in foreign inclusions. Gray color in some diamonds from the Argyle mine
Argyle diamond mine
The Argyle Diamond Mine is a diamond mine located in the East Kimberley region in the remote north of Western Australia. Argyle is the largest diamond producer in the world by volume, although due to the low proportion of gem-quality diamonds, is not the leader by value. It is the only known...
in Australia is often associated with those hydrogen defects, but again, this assignment is yet unproven.
Nickel and cobalt

Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
, optical absorption and photoluminescence spectra, and the concentration of isolated nickel can reach 0.01%. This fact is by all means unusual considering the large difference in size between carbon and transition metal atoms and the superior rigidity of the diamond lattice.
Numerous Ni-related defects have been detected by electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
, optical absorption and photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
, both in synthetic and natural diamonds. Three major structures can be distinguished: substitutional Ni, nickel-vacancy and nickel-vacancy complex decorated by one or more substitutional nitrogen atoms. The "nickel-vacancy" structure, also called "semi-divacancy" is specific for most large impurities in diamond and silicon (e.g., tin in silicon). Its production mechanism is generally accepted as follows: large nickel atom incorporates substitutionally, then expels a nearby carbon (creating a neighboring vacancy), and shifts in-between the two sites.
Although the physical and chemical properties of cobalt and nickel are rather similar, the concentrations of isolated cobalt in diamond are much smaller than those of nickel (parts per billion range). Several defects related to isolated cobalt have been detected by electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
and photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
, but their structure is yet unknown.
Silicon
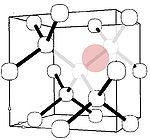
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
. Similar to other large impurities, the major form of silicon in diamond has been identified with a Si-vacancy complex (semi-divacancy site). This center is a deep donor having an ionization energy of 2 eV, and thus again is unsuitable for electronic applications.
Si-vacancies constitute minor fraction of total silicon. It is believed (though no proof exists) that much silicon substitutes for carbon thus becoming invisible to most spectroscopic techniques because silicon and carbon atoms have the same configuration of the outer electronic shells.
Sulfur
Around the year 2000, there was a wave of attempts to dope synthetic CVD diamond films by sulfur aiming at n-type conductivity with low activation energy. Successful reports have been published, but then dismissed as the conductivity was rendered p-type instead of n-type and associated not with sulfur, but with residual boron, which is a highly efficient p-type dopant in diamond.So far (2009), there is only one reliable evidence (through hyperfine interaction structure in electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
) for isolated sulfur defects in diamond. The corresponding center called W31 has been observed in natural type-Ib diamonds in small concentrations (parts per million). It was assigned to a sulfur-vacancy complex – again, as in case of nickel and silicon, a semi-divacancy site.
Intrinsic defects
The easiest way to produce intrinsic defects in diamond is by displacing carbon atoms through irradiation with high-energy particles, such as alpha (helium), beta (electrons) or gamma particles, protons, neutrons, ions, etc. The irradiation can occur in the laboratory or in the nature (see Diamond enhancement – Irradiation); it produces primary defects named frenkel defectFrenkel defect
The Frenkel Defect is shown by ionic solids. The smaller ion is displaced from its lattice position to an interstitial site. It creates a vacancy defect at its original site and an interstitial defect at its new location.-Definition:...
s (carbon atoms knocked off their normal lattice sites to interstitial sites) and remaining lattice vacancies. An important difference between the vacancies and interstitials in diamond is that whereas interstitials are mobile during the irradiation, even at liquid nitrogen temperatures, however vacancies start migrating only at temperatures ~700 0C.
Vacancies and interstitials can also be produced in diamond by plastic deformation, though in much smaller concentrations.
Isolated carbon interstitial
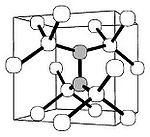
Interstitial defect
Interstitials are a variety of crystallographic defects, i.e. atoms which occupy a site in the crystal structure at which there is usually not an atom, or two or more atoms sharing one or more lattice sites such that the number of atoms is larger than the number of lattice sites.They are generally...
has never been observed in diamond and is considered unstable. Its interaction with a regular carbon lattice atom produces a "split-interstitial", a defect where two carbon atoms share a lattice site and are covalently bonded with the carbon neighbors. This defect has been thoroughly characterized by electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
(R2 center) and optical absorption, and unlike most other defects in diamond, it does not produce photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
.
Interstitial complexes
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
(R1 and O3 centers), optical absorption and photoluminescence.
Vacancy-Interstitial complexes
Most high-energy particles, beside displacing carbon atom from the lattice site, also pass it enough surplus energy for a rapid migration through the lattice. However, when relatively gentle gamma irradiation is used, this extra energy is minimal. Thus the interstitials remain near the original vacancies and form vacancy-interstitials pairs identified through optical absorption.Vacancy-di-interstitial pairs have been also produced, though by electron irradiation and through a different mechanism: Individual interstitials migrate during the irradiation and aggregate to form di-interstitials; this process occurs preferentially near the lattice vacancies.
Isolated vacancy

The vacancy behaves as a deep electron donor/acceptor, whose electronic properties depend on the charge state. The energy level for the +/0 states is at 0.6 eV and for the 0/- states is at 2.5 eV above the valence band
Valence band
In solids, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature....
.
Multivacancy complexes
Upon annealing of pure diamond at ~700 0C, vacancies migrate and form divacancies, characterized by optical absorption and electron paramagnetic resonanceElectron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
.
Similar to single interstitials, divacancies do not produce photoluminescence. Divacancies, in turn, anneal out at ~900 0C creating multivacancy chains detected by EPR and presumably hexavacancy rings. The latter should be invisible to most spectroscopies, and indeed, they have not been detected thus far. Annealing of vacancies changes diamond color from green to yellow-brown. Similar mechanism (vacancy aggregation) is also believed to cause brown color of plastically deformed natural diamonds.
Dislocations
DislocationDislocation
In materials science, a dislocation is a crystallographic defect, or irregularity, within a crystal structure. The presence of dislocations strongly influences many of the properties of materials...
s are the most common structural defect in natural diamond. The two major types of dislocations are the glide set, in which bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
break between layers of atoms with different indices (those not lying directly above each other) and the shuffle set, in which the breaks occur between atoms of the same index. The dislocations produce dangling bonds which introduce energy levels into the band gap, enabling the absorption of light. Broadband blue photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
has been reliably identified with dislocations by direct observation in an electron microscope
Electron microscope
An electron microscope is a type of microscope that uses a beam of electrons to illuminate the specimen and produce a magnified image. Electron microscopes have a greater resolving power than a light-powered optical microscope, because electrons have wavelengths about 100,000 times shorter than...
, however, it was noted that not all dislocations are luminescent, and there is no correlation between the dislocation type and the parameters of the emission.
Platelets

Optical microscope
The optical microscope, often referred to as the "light microscope", is a type of microscope which uses visible light and a system of lenses to magnify images of small samples. Optical microscopes are the oldest design of microscope and were possibly designed in their present compound form in the...
. For a long time, platelets were tentatively associated with large nitrogen complexes — nitrogen sinks produced as a result of nitrogen aggregation at high temperatures of the diamond synthesis. However, direct measurement of nitrogen in the platelets by EELS (an analytical technique of electron microscopy) revealed very little nitrogen. The currently accepted model of platelets is a large regular array of carbon interstitials.
Platelets produce sharp absorption peaks at 1359–1375 and 330 cm−1 in IR absorption spectra; remarkably, the position of the first peak depends on the platelet size. As with dislocations, a broad photoluminescence centered at ~1000 nm was associated with platelets by direct observation in an electron microscope. By studying this luminescence, it was deduced that platelets have a "bandgap" of ~1.7 eV.
Voidites
Octahedron
In geometry, an octahedron is a polyhedron with eight faces. A regular octahedron is a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex....
nanometer-sized clusters present in many natural diamonds, as revealed by electron microscopy. Laboratory experiments demonstrated that annealing of type-IaB diamond at high temperatures and pressures (>2600 0C) results in break-up of the platelets and formation of dislocation loops and voidites, i.e. that voidites are a result of thermal degradation of platelets. Contrary to platelets, voidites do contain much nitrogen, in the molecular form.
Interaction between intrinsic and extrinsic defects
Extrinsic and intrinsic defects can interact producing new defect complexes. Such interaction usually occurs if a diamond containing extrinsic defects (impurities) is either plastically deformed or is irradiated and annealed.
Nitrogen-vacancy center
The nitrogen-vacancy center is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual N-V center...
, which can be neutral or negatively charged; the negatively charged state has potential applications in quantum computing. A and B centers upon trapping a vacancy create corresponding 2N-V (H3 and H2 centers, where H2 is simply a negatively charged H3 center) and the neutral 4N-2V (H4 center). The H2, H3 and H4 centers are important because they are present in many natural diamonds and their optical absorption can be strong enough to alter the diamond color (H3 or H4 – yellow, H2 – green).
Boron interacts with carbon interstitials forming a neutral boron–interstitial complex with a sharp optical absorption at 0.552 eV (2250 nm). No evidence is known so far (2009) for complexes of boron and vacancy.
In contrast, silicon does react with vacancies, creating the described above optical absorption at 738 nm. The assumed mechanism is trapping of migrating vacancy by substitutional silicon resulting in the Si-V (semi-divacancy) configuration.
A similar mechanism is expected for nickel, for which both substitutional and semi-divacancy configurations are reliably identified (see subsection "nickel and cobalt" above). In an unpublished study, diamonds rich in substitutional nickel were electron irradiated and annealed, with following careful optical measurements performed after each annealing step, but no evidence for creation or enhancement of Ni-vacancy centers was obtained.
See also
- Chemical vapor deposition of diamondChemical vapor deposition of diamondChemical vapor deposition of diamond or CVD is a method of producing synthetic diamond by creating the circumstances necessary for carbon atoms in a gas to settle on a substrate in crystalline form....
- Crystallographic defectCrystallographic defectCrystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
- Diamond colorDiamond colorA chemically pure and structurally perfect diamond is perfectly transparent with no hue, or color. However, in reality almost no gem-sized natural diamonds are absolutely perfect. The color of a diamond may be affected by chemical impurities and/or structural defects in the crystal lattice...
- Diamond enhancementDiamond enhancementDiamond enhancements are specific treatments, performed on natural diamonds , which are designed to improve the gemological characteristics — and therefore the value — of the stone in one or more ways...
- Gemstone irradiationGemstone irradiationThe gemstone irradiation is a process in which a gemstone is artificially irradiated in order to enhance its optical properties. High levels of ionizing radiation can change the atomic structure of the gemstone's crystal lattice, which in turn alters the optical properties within it...
- Material properties of diamondMaterial properties of diamondDiamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. Diamond is an optically isotropic crystal that is transparent to opaque. Owing to its strong covalent bonding, diamond is the hardest naturally occurring material...
- Nitrogen-vacancy centerNitrogen-vacancy centerThe nitrogen-vacancy center is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual N-V center...
- Synthetic diamondSynthetic diamondSynthetic diamond is diamond produced in a technological process; as opposed to natural diamond, which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond, denoting the production method, High-Pressure High-Temperature synthesis and Chemical...

