Valence band
Encyclopedia
In solid
s, the valence band is the highest range of electron
energies
in which electrons are normally present at absolute zero
temperature.
.png) The valence electrons are bound to individual atom
The valence electrons are bound to individual atom
s, as opposed to conduction electrons (found in conductors
and semiconductor
s), which can move freely within the atomic lattice of the material. On a graph of the electronic band structure
of a material, the valence band is located below the conduction band
, separated from it in insulators and semiconductors by a band gap
. In metal
s, the conduction band has no energy gap separating it from the valence band.
 To understand the concept of a valence band, it is important to consider the atomic structure of a metal first. For example lithium
To understand the concept of a valence band, it is important to consider the atomic structure of a metal first. For example lithium
(Li) atoms with electronic configuration 1s22s1 can form only one covalent bond
. However, when forming a bulk metal, Li atoms come to a resonance
structure by taking 1 electron from its neighbouring Li atom and the resultant electronic configuration becomes 1s22s12p1 (e-). As a result of this electron sharing, its neighbouring Li atom loses an electron and comes to an electronic configuration of 1s2 (e+). The Li (e-) atoms now gain the capability to form two covalent bonds thus can form a bulk metal. Inside the metal Li (e+) remain alone (not bonded) but nullify the negative charges of the neighbouring Li atoms thus forming a lithium metal matrix.
In a three-dimensional (3D) metal structure of Li, the molecular orbital
formation starts from the lower energy level orbitals, i.e. first 1s, then 2s, then 2p. The molecular bond formation is a rapid process and as a result it is seen that 2s orbitals come to totally filled condition whereas the 2p orbitals only partially filled during that time span, and the remaining part of the 2p orbitals remain empty (no electron). In between there is an overlapped zone of totally filled 2s and totally filled 2p orbitals, called overlapped zone.
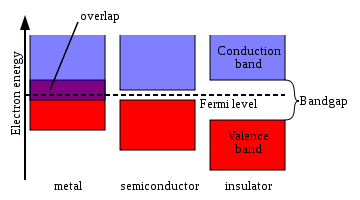
For any metal this is the rule of forming a metal from its atoms. The totally filled orbitals with highest range of electron energies form valence band (the filled 2s orbitals in the example of Li), the empty orbital with no electrons is called the conduction band
(the empty region of 2p orbitals in the example of Li).
The overlapping depends on the interatomic distance (rd) and also on the energy level of the orbitals. If (rd) is large or the orbitals are of large energy level then there may be small overlapping or no overlapping leaving a band gap
(Eg).
The electrical conductivity of a metal depends on its capability to flow electrons from valence band to conduction band. Hence in case of a metal with large overlapped region the electrical conductivity is high along with good metallic property. If there is a small forbidden zone then the flow of electron from valence to conduction band is only possible if an external energy (thermal etc.) is supplied and these groups with small Eg are called semiconductors. If the Eg is sufficiently high then flow of electron from valence to conduction band become negligible under normal conditions, these groups are called Insulators.
Semiconductors and insulators owe their low conductivity to the properties of the valence band in those materials. The number of electrons is precisely equal to the number of states available up to the top of the valence band. There are no available states in the band gap. This means that when an electric field
is applied, the electrons cannot increase their energy (i.e., accelerate) because there are no states available to the electrons where they would be moving faster than they are already going.
There is some conductivity in insulators, however. This is due to thermal excitation—some of the electrons get enough energy to jump the band gap in one go. Once they are in the conduction band, they can conduct electricity, as can the hole
they left behind in the valence band. The hole is an empty state that allows electrons in the valence band some degree of freedom.
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s, the valence band is the highest range of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
energies
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
in which electrons are normally present at absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
temperature.
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s, as opposed to conduction electrons (found in conductors
Electrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
and semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
s), which can move freely within the atomic lattice of the material. On a graph of the electronic band structure
Electronic band structure
In solid-state physics, the electronic band structure of a solid describes those ranges of energy an electron is "forbidden" or "allowed" to have. Band structure derives from the diffraction of the quantum mechanical electron waves in a periodic crystal lattice with a specific crystal system and...
of a material, the valence band is located below the conduction band
Conduction band
In the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
, separated from it in insulators and semiconductors by a band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
. In metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s, the conduction band has no energy gap separating it from the valence band.

Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
(Li) atoms with electronic configuration 1s22s1 can form only one covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
. However, when forming a bulk metal, Li atoms come to a resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
structure by taking 1 electron from its neighbouring Li atom and the resultant electronic configuration becomes 1s22s12p1 (e-). As a result of this electron sharing, its neighbouring Li atom loses an electron and comes to an electronic configuration of 1s2 (e+). The Li (e-) atoms now gain the capability to form two covalent bonds thus can form a bulk metal. Inside the metal Li (e+) remain alone (not bonded) but nullify the negative charges of the neighbouring Li atoms thus forming a lithium metal matrix.
In a three-dimensional (3D) metal structure of Li, the molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
formation starts from the lower energy level orbitals, i.e. first 1s, then 2s, then 2p. The molecular bond formation is a rapid process and as a result it is seen that 2s orbitals come to totally filled condition whereas the 2p orbitals only partially filled during that time span, and the remaining part of the 2p orbitals remain empty (no electron). In between there is an overlapped zone of totally filled 2s and totally filled 2p orbitals, called overlapped zone.
For any metal this is the rule of forming a metal from its atoms. The totally filled orbitals with highest range of electron energies form valence band (the filled 2s orbitals in the example of Li), the empty orbital with no electrons is called the conduction band
Conduction band
In the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
(the empty region of 2p orbitals in the example of Li).
The overlapping depends on the interatomic distance (rd) and also on the energy level of the orbitals. If (rd) is large or the orbitals are of large energy level then there may be small overlapping or no overlapping leaving a band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
(Eg).
The electrical conductivity of a metal depends on its capability to flow electrons from valence band to conduction band. Hence in case of a metal with large overlapped region the electrical conductivity is high along with good metallic property. If there is a small forbidden zone then the flow of electron from valence to conduction band is only possible if an external energy (thermal etc.) is supplied and these groups with small Eg are called semiconductors. If the Eg is sufficiently high then flow of electron from valence to conduction band become negligible under normal conditions, these groups are called Insulators.
Semiconductors and insulators owe their low conductivity to the properties of the valence band in those materials. The number of electrons is precisely equal to the number of states available up to the top of the valence band. There are no available states in the band gap. This means that when an electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
is applied, the electrons cannot increase their energy (i.e., accelerate) because there are no states available to the electrons where they would be moving faster than they are already going.
There is some conductivity in insulators, however. This is due to thermal excitation—some of the electrons get enough energy to jump the band gap in one go. Once they are in the conduction band, they can conduct electricity, as can the hole
Electron hole
An electron hole is the conceptual and mathematical opposite of an electron, useful in the study of physics, chemistry, and electrical engineering. The concept describes the lack of an electron at a position where one could exist in an atom or atomic lattice...
they left behind in the valence band. The hole is an empty state that allows electrons in the valence band some degree of freedom.
See also
- Electrical conduction for more information about conduction in solids, and another description of band structure.
- Conduction bandConduction bandIn the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
- Electronic band structureElectronic band structureIn solid-state physics, the electronic band structure of a solid describes those ranges of energy an electron is "forbidden" or "allowed" to have. Band structure derives from the diffraction of the quantum mechanical electron waves in a periodic crystal lattice with a specific crystal system and...
- Fermi seaFermi liquidFermi liquid theory is a theoretical model of interacting fermions that describes the normal state of most metals at sufficiently low temperatures. The interaction between the particles of the many-body system does not need to be small...

