
Cationic polymerization
Encyclopedia
Cationic polymerization is a type of chain growth polymerization in which a cationic initiator transfers charge to a monomer
which becomes reactive. This reactive monomer goes on to react similarly with other monomers to form a polymer. Poly(isobutyrene) used in inner tubes is the only polymer being commercially produced via cationic polymerization.
The types of monomers necessary for cationic polymerization are limited to olefins with electron-donating substituents and heterocycles. Similar to anionic polymerization
reactions, cationic polymerization reactions are very sensitive to the type of solvent used. Specifically, the ability of a solvent to form free ions will dictate the reactivity of the propagating cationic chain.
Cationic polymerization is used in the production of polyisobutylene and poly(N-vinylcarbazole) (PVK).
and heterocyclic
monomers. Cationic polymerization of both types of monomers occurs only if the overall reaction is thermally favorable. In the case of olefins, this is due to isomerization of the monomer double bond; for heterocycles, this is due to release of monomer ring strain and, in some cases, isomerization of repeating units. Monomers for cationic polymerization are nucleophilic and form a stable cation
upon polymerization.
enough to attack electrophilic
initiators or growing polymer chains. At the same time, these electron-donating groups attached to the monomer must be able to stabilize the resulting cationic charge for further polymerization. Some reactive olefin monomers are shown below in order of decreasing reactivity, with heteroatom
groups being more reactive than alkyl or aryl groups. Note, however, that the reactivity of the carbenium ion formed is the opposite of the monomer reactivity.
s, lactam
s, and cyclic amine
s. Upon addition of an initiator, cyclic monomers go on to form linear polymers. The reactivity of heterocyclic monomers depends on their ring strain. Monomers with large ring strain, such as oxirane
, are more reactive than 1,3-dioxepane which has considerably less ring strain. Rings that are six-membered and larger are less likely to polymerize due to lower ring strain.
is generated from which the polymer chain is made. The counterion should be non-nucleophilic, otherwise the reaction is terminated instantaneously. There are a variety of initiators available for cationic polymerization, and some of them require a coinitiator to generate the needed cationic species.
(A–) produced must be weakly nucleophilic so as to prevent early termination due to combination with the protonated olefin. Common acids used are phosphoric
, sulfuric
, fluro-, and triflic acids
. Only low molecular weight polymers are formed with these initiators.
, alcohol
s, or even a carbocation donor such as an ester
or an anhydride. In these systems the Lewis acid is referred to as a coinitiator while the cation source is the initiator. Upon reaction of the initiator with the coinitiator, an intermediate complex is formed which then goes on to react with the monomer unit. The counterion produced by the initiator-coinitiator complex is less nucleophilic than that of the Brønsted acid A– counterion. Halogens, such as chlorine
and bromine
, can also initiate cationic polymerization upon addition of the more active Lewis acids.
and tropylium cations.
can form a radical-cation pair that can then react with a monomer to start cationic polymerization. Control of the radical-cation pairs are difficult and often depend on the monomer and reaction conditions. Formation of radical
and anionic species are often observed.
 ) is based upon the activation energies for the initiation (
) is based upon the activation energies for the initiation (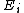 ), propagation (
), propagation (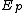 ), and termination (
), and termination (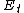 ) steps:
) steps:
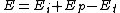
Generally,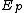 is less than
is less than  and
and 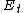 , meaning the overall activation energy
, meaning the overall activation energy
is negative. When this is the case, a decrease in temperature leads to an increase in the rate of propagation. The converse is true when the overall activation energy is positive.
Chain length is also affected by temperature. Low reaction temperatures, in the range of 170–190 K, are preferred for producing longer chains. This comes as a result of the activation energy for termination and other side reactions being larger than the activation energy for propagation. As the temperature is raised, the energy barrier for the termination reaction is overcome, causing shorter chains to be produced during the polymerization process.
The association is strongest as a covalent bond and weakest when the pair exists as free ions. In cationic polymerization, the ions tend to be in equilibrium between an ion pair (either tight or solvent-separated) and free ions. The more polar the solvent used in the reaction, the better the solvation and separation of the ions. Since free ions are more reactive than ion pairs, the rate of propagation is faster in more polar solvents.
The size of the counterion is also a factor. A smaller counterion, with a higher charge density, will have stronger electrostatic interactions with the carbenium ion than will a larger counterion which has a lower charge density. Further, a smaller counterion is more easily solvated by a polar solvent than a counterion with low charge density. The result is increased propagation rate with increased solvating capability of the solvent.
The second method involves hydrogen abstraction from the active chain end to the monomer. This terminates the growing chain and also forms a new active carbenium ion-counterion complex which can continue to propagate, thus keeping the kinetic chain intact.
s, hence end-capping of the polymer chains is often necessary to prevent depolymerization.
of the polymerization. The reaction equations for initiation, propagation, termination, and chain transfer can be written in a general form:

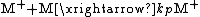
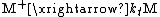

In which I+ is the initiator, M is the monomer, M+ is the propagating center, and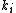 ,
, 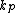 ,
, 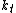 , and
, and 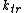 are the rate constants for initiation, propagation, termination, and chain transfer, respectively. For simplicity, counterions are not shown in the above reaction equations and only chain transfer to monomer is considered. The resulting rate equations are as follows, where brackets denote concentrations:
are the rate constants for initiation, propagation, termination, and chain transfer, respectively. For simplicity, counterions are not shown in the above reaction equations and only chain transfer to monomer is considered. The resulting rate equations are as follows, where brackets denote concentrations:
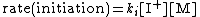
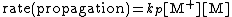
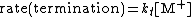
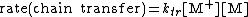
Assuming steady-state conditions
, i.e. the rate of initiation = rate of termination:
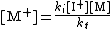
This equation for [M+] can then be used in the equation for the rate of propagation:

From this equation, it is seen that propagation rate increases with increasing monomer and initiator concentration.
The degree of polymerization
,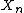 , can be determined from the rates of propagation and termination:
, can be determined from the rates of propagation and termination:

If chain transfer rather than termination is dominant, the equation for becomes
becomes
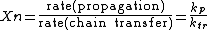
and butyl rubber
. These polymers have a variety of applications from adhesives and sealants to protective gloves and pharmaceutical stoppers. The reaction conditions for the synthesis of each type of isobutylene product vary depending on the desired molecular weight and what type(s) of monomer(s) is used. The conditions most commonly used to form low molecular weight (5–10 x 104 Da) polyisobutylene are initiation with AlCl3, BF3, or TiCl4 at a temperature range of −40 to 10 °C. These low molecular weight polyisobutylene polymers are used for caulking and as sealants. High molecular weight PIBs are synthesized at much lower temperatures of −100 to −90 °C and in a polar medium of methylene chloride. These polymers are used to make uncrosslinked rubber products and are additives for certain thermoplasts. Another characteristic of high molecular weight PIB is its low toxicity which allows it to be used as a base for chewing gum. The main chemical companies that produce polyisobutylene are Esso
, ExxonMobil
, and BASF
.
Butyl rubber, in contrast to PIB, is a copolymer in which the monomers isobutylene
(~98%) and isoprene
(2%) are polymerized in a process similar to high molecular weight PIBs. Butyl rubber polymerization is carried out as a continuous process with AlCl3 as the initiator. Its low gas permeability and good resistance to chemicals and aging make it useful for a variety of applications such as protective gloves, electrical cable insulation, and even basketballs. Large scale production of butyl rubber started during World War II, and roughly 1 billion pounds/year are produced in the U.S. today.
Polybutene is another copolymer, containing roughly 80% isobutylene and 20% other butenes (usually 1-butene
). The production of these low molecular weight polymers (300–2500 Da) is done within a large range of temperatures (−45 to 80 °C) with AlCl3 or BF3. Depending on the molecular weight of these polymers, they can be used as adhesives, sealants, plasticizers, additives for transmission fluids, and a variety of other applications. These materials are low-cost and are made by a variety of different companies including BP
Chemicals, Esso, and BASF.
Other polymers formed by cationic polymerization are homopolymers and copolymers of polyterpenes, such as pinene
s (plant-derived products), that are used as tackyfiers. In the field of heterocycles, 1,3,5-trioxane
is copolymerized with small amounts of ethylene oxide to form the highly crystalline polyoxymethylene plastic. Also, the homopolymerization of alkyl vinyl ethers is achieved only by cationic polymerization.
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
which becomes reactive. This reactive monomer goes on to react similarly with other monomers to form a polymer. Poly(isobutyrene) used in inner tubes is the only polymer being commercially produced via cationic polymerization.
The types of monomers necessary for cationic polymerization are limited to olefins with electron-donating substituents and heterocycles. Similar to anionic polymerization
Anionic addition polymerization
Anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of vinyl monomers with strong electronegative groups. This polymerization is carried out through a carbanion active species. Like all addition polymerizations, it...
reactions, cationic polymerization reactions are very sensitive to the type of solvent used. Specifically, the ability of a solvent to form free ions will dictate the reactivity of the propagating cationic chain.
Cationic polymerization is used in the production of polyisobutylene and poly(N-vinylcarbazole) (PVK).
Monomers
Monomer scope for cationic polymerization is limited to two main types: olefinsAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and heterocyclic
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
monomers. Cationic polymerization of both types of monomers occurs only if the overall reaction is thermally favorable. In the case of olefins, this is due to isomerization of the monomer double bond; for heterocycles, this is due to release of monomer ring strain and, in some cases, isomerization of repeating units. Monomers for cationic polymerization are nucleophilic and form a stable cation
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
upon polymerization.
Olefins
Cationic polymerization of olefin monomers occurs with olefins that contain electron-donating substituents. These electron-donating groups make the olefin nucleophilicNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
enough to attack electrophilic
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
initiators or growing polymer chains. At the same time, these electron-donating groups attached to the monomer must be able to stabilize the resulting cationic charge for further polymerization. Some reactive olefin monomers are shown below in order of decreasing reactivity, with heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
groups being more reactive than alkyl or aryl groups. Note, however, that the reactivity of the carbenium ion formed is the opposite of the monomer reactivity.
Heterocyclic monomers
Heterocyclic monomers that are cationically polymerized are lactoneLactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s, lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
s, and cyclic amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. Upon addition of an initiator, cyclic monomers go on to form linear polymers. The reactivity of heterocyclic monomers depends on their ring strain. Monomers with large ring strain, such as oxirane
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
, are more reactive than 1,3-dioxepane which has considerably less ring strain. Rings that are six-membered and larger are less likely to polymerize due to lower ring strain.
Initiation
Initiation is the first step in cationic polymerization. During initiation, a carbenium ionCarbenium ion
A carbenium ion is a carbocation of the trivalent and classical type R3C+. It is one of two types of carbocation, the other being a carbonium ion. In older literature a carbocation of the type R3C+ may still be referred to as a carbonium ion, a term that is used now for five-coordinate carbon...
is generated from which the polymer chain is made. The counterion should be non-nucleophilic, otherwise the reaction is terminated instantaneously. There are a variety of initiators available for cationic polymerization, and some of them require a coinitiator to generate the needed cationic species.
Classical protonic acids
Strong protic acids can be used to form a cationic initiating species. High concentrations of the acid are needed in order to produce sufficient quantities of the cationic species. The counterionCounterion
A counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt the sodium cation is the counterion for the chlorine anion and vice versa.In a charged transition metal complex, a simple A counterion is the ion that accompanies an ionic species in...
(A–) produced must be weakly nucleophilic so as to prevent early termination due to combination with the protonated olefin. Common acids used are phosphoric
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
, sulfuric
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, fluro-, and triflic acids
Trifluoromethanesulfonic acid
Trifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification.-Properties:Triflic acid is a hygroscopic, colorless...
. Only low molecular weight polymers are formed with these initiators.
Lewis acids/Friedel-Crafts catalysts
Lewis acids are the most common compounds used for initiation of cationic polymerization. The more popular Lewis acids are SnCl4, AlCl3, BF3, and TiCl4. Although these Lewis acids alone are able to induce polymerization, the reaction occurs much faster with a suitable cation source. The cation source can be waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, or even a carbocation donor such as an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
or an anhydride. In these systems the Lewis acid is referred to as a coinitiator while the cation source is the initiator. Upon reaction of the initiator with the coinitiator, an intermediate complex is formed which then goes on to react with the monomer unit. The counterion produced by the initiator-coinitiator complex is less nucleophilic than that of the Brønsted acid A– counterion. Halogens, such as chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
and bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
, can also initiate cationic polymerization upon addition of the more active Lewis acids.
Carbenium ion salts
Stable carbenium ions are used to initiate chain growth of only the most reactive olefins and are known to give well defined structures. These initiators are most often used in kinetic studies due to the ease of being able to measure the disappearance of the carbenium ion absorbance. Common carbenium ions are tritylTriphenylmethyl chloride
Triphenylmethyl chloride or trityl chloride is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.-Preparation:Triphenylmethyl chloride is commercially available...
and tropylium cations.
Ionizing radiation
Ionizing radiationIonizing radiation
Ionizing radiation is radiation composed of particles that individually have sufficient energy to remove an electron from an atom or molecule. This ionization produces free radicals, which are atoms or molecules containing unpaired electrons...
can form a radical-cation pair that can then react with a monomer to start cationic polymerization. Control of the radical-cation pairs are difficult and often depend on the monomer and reaction conditions. Formation of radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
and anionic species are often observed.
Propagation
Propagation proceeds via addition of monomer to the active species, i.e. the carbenium ion. The monomer is added to the growing chain in a head-to-tail fashion; in the process, the cationic end group is regenerated to allow for the next round of monomer addition.Effect of temperature
The temperature of the reaction has an effect on the rate of propagation. The overall activation energy for the polymerization ( ) is based upon the activation energies for the initiation (
) is based upon the activation energies for the initiation (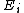 ), propagation (
), propagation (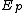 ), and termination (
), and termination (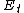 ) steps:
) steps: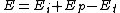
Generally,
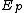 is less than
is less than  and
and 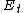 , meaning the overall activation energy
, meaning the overall activation energyActivation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
is negative. When this is the case, a decrease in temperature leads to an increase in the rate of propagation. The converse is true when the overall activation energy is positive.
Chain length is also affected by temperature. Low reaction temperatures, in the range of 170–190 K, are preferred for producing longer chains. This comes as a result of the activation energy for termination and other side reactions being larger than the activation energy for propagation. As the temperature is raised, the energy barrier for the termination reaction is overcome, causing shorter chains to be produced during the polymerization process.
Effect of solvent and counterion
The solvent and the counterion (the gegen ion) have a significant effect on the rate of propagation. The counterion and the carbenium ion can have different associations, ranging from a covalent bond, tight ion pair (unseparated), solvent-separated ion pair (partially separated), and free ions (completely dissociated).The association is strongest as a covalent bond and weakest when the pair exists as free ions. In cationic polymerization, the ions tend to be in equilibrium between an ion pair (either tight or solvent-separated) and free ions. The more polar the solvent used in the reaction, the better the solvation and separation of the ions. Since free ions are more reactive than ion pairs, the rate of propagation is faster in more polar solvents.
The size of the counterion is also a factor. A smaller counterion, with a higher charge density, will have stronger electrostatic interactions with the carbenium ion than will a larger counterion which has a lower charge density. Further, a smaller counterion is more easily solvated by a polar solvent than a counterion with low charge density. The result is increased propagation rate with increased solvating capability of the solvent.
Termination
Termination generally occurs via unimolecular rearrangement with the counterion. In this process, an anionic fragment of the counterion combines with the propagating chain end. This not only inactivates the growing chain, but it also terminates the kinetic chain by reducing the concentration of the initiator-coinitiator complex.Chain transfer
Chain transfer can take place in two ways. One method of chain transfer is hydrogen abstraction from the active chain end to the counterion. In this process, the growing chain is terminated, but the initiator-coinitiator complex is regenerated to initiate more chains.The second method involves hydrogen abstraction from the active chain end to the monomer. This terminates the growing chain and also forms a new active carbenium ion-counterion complex which can continue to propagate, thus keeping the kinetic chain intact.
Cationic ring-opening polymerization
Cationic ring-opening polymerization follows the same mechanistic steps of initiation, propagation, and termination. However, in this polymerization reaction, the monomer units are cyclic in comparison to the resulting polymer chains which are linear. The linear polymers produced can have low ceiling temperatureCeiling temperature
Ceiling temperature is a measure of the tendency of polymers to revert to their monomers. When a polymer is at its ceiling temperature, the rate of polymerization and depolymerization of the polymer are equal. Generally, the ceiling temperature of a given polymer is correlated to the steric...
s, hence end-capping of the polymer chains is often necessary to prevent depolymerization.
Kinetics
The rate of propagation and the degree of polymerization can be determined from an analysis of the kineticsChemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
of the polymerization. The reaction equations for initiation, propagation, termination, and chain transfer can be written in a general form:

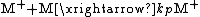
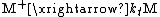

In which I+ is the initiator, M is the monomer, M+ is the propagating center, and
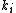 ,
, 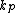 ,
, 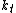 , and
, and 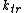 are the rate constants for initiation, propagation, termination, and chain transfer, respectively. For simplicity, counterions are not shown in the above reaction equations and only chain transfer to monomer is considered. The resulting rate equations are as follows, where brackets denote concentrations:
are the rate constants for initiation, propagation, termination, and chain transfer, respectively. For simplicity, counterions are not shown in the above reaction equations and only chain transfer to monomer is considered. The resulting rate equations are as follows, where brackets denote concentrations: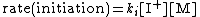
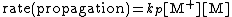
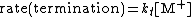
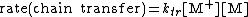
Assuming steady-state conditions
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
, i.e. the rate of initiation = rate of termination:
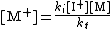
This equation for [M+] can then be used in the equation for the rate of propagation:

From this equation, it is seen that propagation rate increases with increasing monomer and initiator concentration.
The degree of polymerization
Degree of polymerization
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.For a homopolymer, there is only one type of monomeric unit andthe number-average degree of polymerization is given by...
,
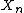 , can be determined from the rates of propagation and termination:
, can be determined from the rates of propagation and termination:
If chain transfer rather than termination is dominant, the equation for
 becomes
becomes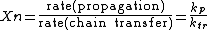
Living polymerization
In 1984, Higashimura and Sawamoto reported the first living cationic polymerization for alkyl vinyl ethers. This type of polymerization has allowed for the control of well-defined polymers. A key characteristic of living cationic polymerization is that termination is essentially eliminated, thus the cationic chain growth continues until all monomer is consumed.Commercial applications
The largest commercial application of cationic polymerization is in the production of polyisobutylene (PIB) products which include polybutenePolybutene
Polybutene and polyisobutylene are liquid oligomers widely used as plasticizers for high-molecular weight polymers, such as polyethylene. They are not to be confused with the high molecular weight polymer polybutene-1.-Properties:...
and butyl rubber
Butyl rubber
Butyl rubber is a synthetic rubber, a copolymer of isobutylene with isoprene. The abbreviation IIR stands for Isobutylene Isoprene Rubber. Polyisobutylene, also known as "PIB" or polyisobutene, n, is the homopolymer of isobutylene, or 2-methyl-1-propene, on which butyl rubber is based...
. These polymers have a variety of applications from adhesives and sealants to protective gloves and pharmaceutical stoppers. The reaction conditions for the synthesis of each type of isobutylene product vary depending on the desired molecular weight and what type(s) of monomer(s) is used. The conditions most commonly used to form low molecular weight (5–10 x 104 Da) polyisobutylene are initiation with AlCl3, BF3, or TiCl4 at a temperature range of −40 to 10 °C. These low molecular weight polyisobutylene polymers are used for caulking and as sealants. High molecular weight PIBs are synthesized at much lower temperatures of −100 to −90 °C and in a polar medium of methylene chloride. These polymers are used to make uncrosslinked rubber products and are additives for certain thermoplasts. Another characteristic of high molecular weight PIB is its low toxicity which allows it to be used as a base for chewing gum. The main chemical companies that produce polyisobutylene are Esso
Esso
Esso is an international trade name for ExxonMobil and its related companies. Pronounced , it is derived from the initials of the pre-1911 Standard Oil, and as such became the focus of much litigation and regulatory restriction in the United States. In 1972, it was largely replaced in the U.S. by...
, ExxonMobil
ExxonMobil
Exxon Mobil Corporation or ExxonMobil, is an American multinational oil and gas corporation. It is a direct descendant of John D. Rockefeller's Standard Oil company, and was formed on November 30, 1999, by the merger of Exxon and Mobil. Its headquarters are in Irving, Texas...
, and BASF
BASF
BASF SE is the largest chemical company in the world and is headquartered in Germany. BASF originally stood for Badische Anilin- und Soda-Fabrik . Today, the four letters are a registered trademark and the company is listed on the Frankfurt Stock Exchange, London Stock Exchange, and Zurich Stock...
.
Butyl rubber, in contrast to PIB, is a copolymer in which the monomers isobutylene
Isobutylene
Isobutylene is a hydrocarbon of significant industrial importance. It is a four-carbon branched alkene , one of the four isomers of butylene. At standard temperature and pressure it is a colorless flammable gas.-Uses:...
(~98%) and isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
(2%) are polymerized in a process similar to high molecular weight PIBs. Butyl rubber polymerization is carried out as a continuous process with AlCl3 as the initiator. Its low gas permeability and good resistance to chemicals and aging make it useful for a variety of applications such as protective gloves, electrical cable insulation, and even basketballs. Large scale production of butyl rubber started during World War II, and roughly 1 billion pounds/year are produced in the U.S. today.
Polybutene is another copolymer, containing roughly 80% isobutylene and 20% other butenes (usually 1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
). The production of these low molecular weight polymers (300–2500 Da) is done within a large range of temperatures (−45 to 80 °C) with AlCl3 or BF3. Depending on the molecular weight of these polymers, they can be used as adhesives, sealants, plasticizers, additives for transmission fluids, and a variety of other applications. These materials are low-cost and are made by a variety of different companies including BP
BP
BP p.l.c. is a global oil and gas company headquartered in London, United Kingdom. It is the third-largest energy company and fourth-largest company in the world measured by revenues and one of the six oil and gas "supermajors"...
Chemicals, Esso, and BASF.
Other polymers formed by cationic polymerization are homopolymers and copolymers of polyterpenes, such as pinene
Pinene
Pinene is a bicyclic monoterpene chemical compound. There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous...
s (plant-derived products), that are used as tackyfiers. In the field of heterocycles, 1,3,5-trioxane
1,3,5-Trioxane
1,3,5-Trioxane, sometimes also called trioxin, is a chemical compound with molecular formula C3H6O3. It is a stable cyclic trimer of formaldehyde, and one of the two trioxane isomers; its molecular backbone consists of a six membered ring with three carbon atoms alternating with three oxygen...
is copolymerized with small amounts of ethylene oxide to form the highly crystalline polyoxymethylene plastic. Also, the homopolymerization of alkyl vinyl ethers is achieved only by cationic polymerization.

