
Ullmann reaction
Encyclopedia
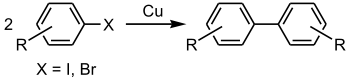
The Ullmann reaction or Ullmann coupling is a coupling reaction
between aryl
halide
s with copper
. The reaction is named after Fritz Ullmann
.
 A typical example is the coupling of 2 o-chloronitrobenzene
A typical example is the coupling of 2 o-chloronitrobenzene
reactants to form 2,2'-dinitrobiphenyl
with a copper - bronze alloy
.
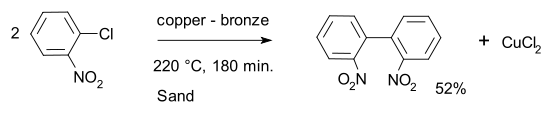 The traditional version of the Ullmann reaction requires harsh reaction conditions, and the reaction has a reputation for erratic yields. Since its discovery some improvements and alternative procedures have been introduced.
The traditional version of the Ullmann reaction requires harsh reaction conditions, and the reaction has a reputation for erratic yields. Since its discovery some improvements and alternative procedures have been introduced.
The reaction mechanism
of the Ullmann reaction is extensively studied. Electron spin resonance rules out a radical
intermediate. The oxidative addition
/ reductive elimination sequence observed with palladium
catalysts is unlikely for copper
because copper(III) is rarely observed. The reaction probably involves the formation of an organocopper compound
(RCuX) which reacts with the other aryl reactant in a nucleophilic aromatic substitution
. Alternative mechanisms do exist such as σ-bond
metathesis .
The classical Ullmann reaction is limited to electron deficient aryl halides and requires harsh reaction conditions. Modern variants of the Ullman reaction employing palladium and nickel have widened the substrate scope of the reaction and rendered reaction conditions more mild. Yields are generally still moderate, however. In organic synthesis
this reaction is often replaced by palladium coupling reactions such as the Heck reaction
, the Hiyama coupling
and the Sonogashira coupling
.
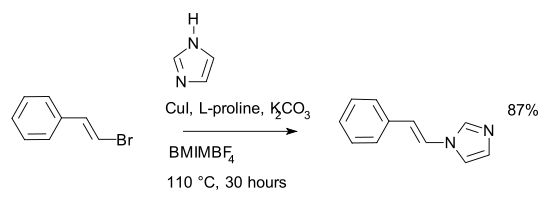
In a variation of the Ullmann reaction, (2-bromovinyl
)-benzene
is reacted with imidazole
in an ionic liquid
, BMIMBF4*, to give N-(2-phenylvinyl)-imidazole. The reaction requires (L)-proline catalysis
.
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
between aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s with copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
. The reaction is named after Fritz Ullmann
Fritz Ullmann
Fritz Ullmann was a German chemist.Ullmann was born in Fürth and started studying chemistry in Nuremberg, but received his PhD of the University of Geneva for work with Carl Gräbe in 1895...
.

Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
reactants to form 2,2'-dinitrobiphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
with a copper - bronze alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
.

The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of the Ullmann reaction is extensively studied. Electron spin resonance rules out a radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
intermediate. The oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
/ reductive elimination sequence observed with palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalysts is unlikely for copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
because copper(III) is rarely observed. The reaction probably involves the formation of an organocopper compound
Organocopper compound
Organocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions...
(RCuX) which reacts with the other aryl reactant in a nucleophilic aromatic substitution
Nucleophilic aromatic substitution
right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
. Alternative mechanisms do exist such as σ-bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
metathesis .
The classical Ullmann reaction is limited to electron deficient aryl halides and requires harsh reaction conditions. Modern variants of the Ullman reaction employing palladium and nickel have widened the substrate scope of the reaction and rendered reaction conditions more mild. Yields are generally still moderate, however. In organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
this reaction is often replaced by palladium coupling reactions such as the Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
, the Hiyama coupling
Hiyama coupling
In organic chemistry, a Hiyama coupling is a palladium or nickel-catalyzed cross coupling reaction of organosilanes with organic halides or triflates. Hiyama couplings were first reported by Yasuo Hatanaka and Tamejiro Hiyama in 1988....
and the Sonogashira coupling
Sonogashira coupling
In organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...
.
In a variation of the Ullmann reaction, (2-bromovinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
)-benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
is reacted with imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
in an ionic liquid
Ionic liquid
An ionic liquid is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ILs are largely made...
, BMIMBF4*, to give N-(2-phenylvinyl)-imidazole. The reaction requires (L)-proline catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
.

Note
- * BMIMBF4 stands for the ionic liquidIonic liquidAn ionic liquid is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ILs are largely made...
, 1-butyl-3-methylimidazolium tetrafluoroborate

