
Thermal oxidation
Encyclopedia

Microfabrication
Microfabrication is the term that describes processes of fabrication of miniature structures, of micrometre sizes and smaller. Historically the earliest microfabrication processes were used for integrated circuit fabrication, also known as "semiconductor manufacturing" or "semiconductor device...
, thermal oxidation is a way to produce a thin layer of oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
(usually silicon dioxide
Silicon dioxide
The chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
) on the surface of a wafer
Wafer (electronics)
A wafer is a thin slice of semiconductor material, such as a silicon crystal, used in the fabrication of integrated circuits and other microdevices...
. The technique forces an oxidizing agent to diffuse into the wafer at high temperature and react with it. The rate of oxide growth is often predicted by the Deal-Grove model
Deal-Grove model
The Deal–Grove model mathematically describes the growth of an oxide layer on the surface of a material. In particular, it is used to analyze thermal oxidation of silicon in semiconductor device fabrication...
. Thermal oxidation may be applied to different materials, but this article will only consider oxidation of silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
substrates to produce silicon dioxide
Silicon dioxide
The chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
.
The chemical reaction
Thermal oxidation of silicon is usually performed at a temperature between 800 and 1200°CCelsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
, resulting in so called High Temperature Oxide layer (HTO). It may use either water vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
(usually UHP steam
Ultra High Purity Steam for Oxidation and Annealing
-Introduction:Ultra High Purity Steam, also called clean steam, UHP steam or high purity water vapor, is used in a variety of industrial manufacturing processes that require oxidation or annealing. These processes include oxide layers grow on silicon wafers for the semiconductor industry and for...
) or molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
as the oxidant; it is consequently called either wet or dry oxidation. The reaction is one of the following:
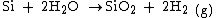
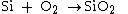
The oxidizing ambient may also contain several percent of hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
(HCl). The chlorine removes metal ions that may occur in the oxide.
Thermal oxide incorporates silicon consumed from the substrate and oxygen supplied from the ambient. Thus, it grows both down into the wafer and up out of it. For every unit thickness of silicon consumed, 2.27 unit thicknesses of oxide will appear. Conversely, if a bare silicon surface is oxidized, 44% of the oxide thickness will lie below the original surface, and 56% above it.
Deal-Grove model
According to the commonly-used Deal-Grove model, the time t required to grow an oxide of thickness Xo, at a constant temperature, on a bare silicon surface, is:
where the constants A and B encapsulate the properties of the reaction and the oxide layer, respectively.
If a wafer that already contains oxide is placed in an oxidizing ambient, this equation must be modified by adding a corrective term τ, the time that would have been required to grow the pre-existing oxide under current conditions. This term may be found using the equation for t above.
Solving the quadratic equation for Xo yields:

Oxidation technology
Most thermal oxidation is performed in furnaceFurnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
s, at temperatures between 800 and 1200°C. A single furnace accepts many wafers at the same time, in a specially designed quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
rack (called a "boat"). Historically, the boat entered the oxidation chamber from the side (this design is called "horizontal"), and held the wafers vertically, beside each other. However, many modern designs hold the wafers horizontally, above and below each other, and load them into the oxidation chamber from below.
Vertical furnaces stand higher than horizontal furnaces, so they may not fit into some microfabrication facilities. However, they help to prevent dust
Dust
Dust consists of particles in the atmosphere that arise from various sources such as soil dust lifted up by wind , volcanic eruptions, and pollution...
contamination. Unlike horizontal furnaces, in which falling dust can contaminate any wafer, vertical furnaces only allow it to fall on the top wafer in the boat.
Vertical furnaces also eliminate an issue that plagued horizontal furnaces: non-uniformity of grown oxide across the wafer. Horizontal furnaces typically have convection currents inside the tube which causes the bottom of the tube to be slightly colder than the top of the tube. As the wafers lie vertically in the tube the convection and the temperature gradient with it causes the top of the wafer to have a thicker oxide than the bottom of the wafer. Vertical furnaces solve this problem by having wafer sitting horizontally, and then having the gas flow in the furnace flowing from top to bottom, significantly dampening any thermal convections.
Vertical furnaces also allow the use of load locks to purge the wafers with nitrogen before oxidation to limit the growth of native oxide on the Si surface.
Oxide quality
Wet oxidation is preferred to dry oxidation for growing thick oxides, because of the higher growth rate. However, fast oxidation leaves more dangling bondDangling bond
In chemistry, a dangling bond is an unsatisfied valence on an immobilised atom.In order to gain enough electrons to fill their valence shells , many atoms will form covalent bonds with other atoms. In the simplest case, that of a single bond, two atoms each contribute one unpaired electron, and the...
s at the silicon interface, which produce quantum states for electrons and allow current to leak along the interface. (This is called a "dirty" interface.) Wet oxidation also yields a lower-density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
oxide, with lower dielectric strength
Dielectric strength
In physics, the term dielectric strength has the following meanings:*Of an insulating material, the maximum electric field strength that it can withstand intrinsically without breaking down, i.e., without experiencing failure of its insulating properties....
.
The long time required to grow a thick oxide in dry oxidation makes this process impractical. Thick oxides are usually grown with a long wet oxidation bracketed by short dry ones (a dry-wet-dry cycle). The beginning and ending dry oxidations produce films of high-quality oxide at the outer and inner surfaces of the oxide layer, respectively.
Mobile metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s can degrade performance of MOSFET
MOSFET
The metal–oxide–semiconductor field-effect transistor is a transistor used for amplifying or switching electronic signals. The basic principle of this kind of transistor was first patented by Julius Edgar Lilienfeld in 1925...
s (sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
is of particular concern). However, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
can immobilize sodium by forming sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
. Chlorine is often introduced by adding hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
or trichloroethylene
Trichloroethylene
The chemical compound trichloroethylene is a chlorinated hydrocarbon commonly used as an industrial solvent. It is a clear non-flammable liquid with a sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.The IUPAC name is...
to the oxidizing medium. Its presence also increases the rate of oxidation.
Other notes
- Thermal oxidation can be performed on selected areas of a wafer, and blocked on others. Areas which are not to be oxidized are covered with a film of silicon nitrideSilicon nitrideSilicon nitride is a chemical compound of silicon and nitrogen. If powdered silicon is heated between 1300° and 1400°C in an atmosphere of nitrogen, trisilicon tetranitride, Si3N4, is formed. The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen...
, which blocks diffusion of oxygen and water vapor. The nitride is removed after oxidation is complete. This process cannot produce sharp features, because lateral (parallel to the surface) diffusion of oxidant molecules under the nitride mask causes the oxide to protrude into the masked area.
- Because impurities dissolveSolvationSolvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
differently in silicon and oxide, a growing oxide will selectively take up or reject dopantDopantA dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
s. This redistribution is governed by the segregation coefficient, which determines how strongly the oxide absorbs or rejects the dopant, and the diffusivityDiffusivityDiffusivity can refer to:*Diffusivity of heat*Diffusivity of mass:** Molecular diffusivity ** Eddy diffusivity*Momentum diffusivity...
.
- The orientation of the silicon crystalCrystalA crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
affects oxidation. A <100> wafer (see Miller indices) oxidizes more slowly than a <111> wafer, but produces an electrically cleaner oxide interface.
- Thermal oxidation of any variety produces a higher-quality oxide, with a much cleaner interface, than chemical vapor depositionChemical vapor depositionChemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
of oxide resulting in Low Temperature Oxide layer (reaction of TEOSTetraethyl orthosilicateTetraethyl orthosilicate is the chemical compound with the formula Si4. Often abbreviated TEOS, this molecule consists of four ethyl groups attached to SiO44- ion, which is called orthosilicate. As an ion in solution, orthosilicate does not exist. Alternatively TEOS can be considered to be the...
at about 600 °C). However, the high temperatures required to produce High Temperature Oxide (HTO) restrict its usability. For instance, in MOSFETMOSFETThe metal–oxide–semiconductor field-effect transistor is a transistor used for amplifying or switching electronic signals. The basic principle of this kind of transistor was first patented by Julius Edgar Lilienfeld in 1925...
processes, thermal oxidation is never performed after the doping for the source and drain terminals is performed, because it would disturb the placement of the dopants.
External links
- Oxide growth time calculator
- Online calculator including deal grove and massoud oxidation models, with pressure and doping effects at: http://www.lelandstanfordjunior.com/thermaloxide.html

