Deal-Grove model
Encyclopedia
The Deal–Grove model mathematically describes the growth of an oxide
layer on the surface of a material. In particular, it is used to analyze thermal oxidation
of silicon
in semiconductor device fabrication. The model was first published in 1965 by Bruce Deal and Andrew Grove
, of Fairchild Semiconductor
.
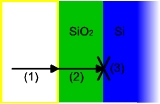
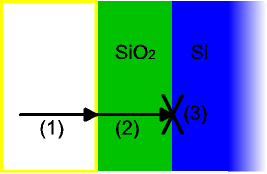 The model assumes that oxidation
The model assumes that oxidation
reaction
occurs at the interface between the oxide and the substrate, rather than between the oxide and the ambient gas
. Thus, it considers three phenomena that the oxidizing species undergoes, in this order:
The model assumes that each of these stages proceeds at a rate proportional to the oxidant's concentration. In the first case, this means Henry's law
; in the second, Fick's law of diffusion
; in the third, a first-order reaction
with respect to the oxidant. It also assumes steady state
conditions, i.e. that transient effects do not appear.
of oxidant through each of the three phases can be expressed in terms of concentrations, material properties, and temperature. By setting the three fluxes equal to each other, they may each be found. In turn, the growth rate may be found readily from the oxidant reaction flux.
In practice, the ambient gas (stage 1) does not limit the reaction rate, so this part of the equation is often dropped. This simplification yields a simple quadratic equation for the oxide thickness. For oxide growing on an initially bare substrate, the thickness Xo at time t is given by the following equation: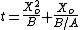
where the constants A and B encapsulate the properties of the reaction and the oxide layer, respectively.
If a wafer that already contains oxide is placed in an oxidizing ambient, this equation must be modified by adding a corrective term τ, the time that would have been required to grow the pre-existing oxide under current conditions. This term may be found using the equation for t above.
Solving the quadratic equation for Xo yields: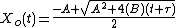
Taking the short and long time limits of the above equation reveals two main modes of operation: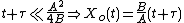
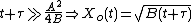
Because they appear in these equations, the quantities B and B/A are often called the quadratic and linear reaction rate constants. They depend exponentially on temperature, like this: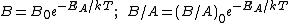
where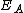 is the activation energy
is the activation energy
and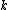 is the Boltzmann Constant in eV.
is the Boltzmann Constant in eV. 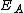 differs from one equation to the other. The following table lists the values of the four parameters for single-crystal
differs from one equation to the other. The following table lists the values of the four parameters for single-crystal
silicon under conditions typically used in industry (low doping
, atmospheric
pressure
). The linear rate constant depends on the orientation of the crystal (usually indicated by the Miller indices of the crystal plane facing the surface). The table gives values for <100> and <111> silicon.
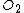 than the model predicts. This phenomenon is not well understood theoretically, but it can be modeled.
than the model predicts. This phenomenon is not well understood theoretically, but it can be modeled.
If the oxide grown in a particular oxidation step will significantly exceed 25 nm, a simple adjustment accounts for the aberrant growth rate. The model yields accurate results for thick oxides if, instead of assuming zero initial thickness (or any initial thickness less than 25 nm), we assume that 25 nm of oxide exists before oxidation begins. However, for oxides near to or thinner than this threshold, more sophisticated models must be used.
Deal-Grove also fails for polycrystalline silicon ("poly-silicon"). First, the random orientation of the crystal grains makes it difficult to choose a value for the linear rate constant. Second, oxidant molecules diffuse rapidly along grain boundaries, so that poly-silicon oxidizes more rapidly than single-crystal silicon.
Dopant
atoms strain the silicon lattice, and make it easier for silicon atoms to bond with incoming oxygen. This effect may be neglected in many cases, but heavily-doped silicon oxidizes significantly faster. The pressure of the ambient gas also affects oxidation rate.
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
layer on the surface of a material. In particular, it is used to analyze thermal oxidation
Thermal oxidation
In microfabrication, thermal oxidation is a way to produce a thin layer of oxide on the surface of a wafer. The technique forces an oxidizing agent to diffuse into the wafer at high temperature and react with it. The rate of oxide growth is often predicted by the Deal-Grove model...
of silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
in semiconductor device fabrication. The model was first published in 1965 by Bruce Deal and Andrew Grove
Andrew Grove
Andrew Stephen Grove , is a Hungarian-born Jewish-American Businessman/ Engineer, Author & a science pioneer in the semiconductor industry. He escaped from Communist-controlled Hungary at the age of 20 and moved to the U.S., where he finished his education...
, of Fairchild Semiconductor
Fairchild Semiconductor
Fairchild Semiconductor International, Inc. is an American semiconductor company based in San Jose, California. Founded in 1957, it was a pioneer in transistor and integrated circuit manufacturing...
.
Physical assumptions

Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
occurs at the interface between the oxide and the substrate, rather than between the oxide and the ambient gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
. Thus, it considers three phenomena that the oxidizing species undergoes, in this order:
- It diffusesDiffusionMolecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
from the bulk of the ambient gas to the surface. - It diffuses through the existing oxide layer to the oxide-substrate interface.
- It reacts with the substrate.
The model assumes that each of these stages proceeds at a rate proportional to the oxidant's concentration. In the first case, this means Henry's law
Henry's law
In physics, Henry's law is one of the gas laws formulated by William Henry in 1803. It states that:An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid...
; in the second, Fick's law of diffusion
Fick's law of diffusion
Fick's laws of diffusion describe diffusion and can be used to solve for the diffusion coefficient, D. They were derived by Adolf Fick in the year 1855.- Fick's first law :...
; in the third, a first-order reaction
First-order reaction
First-order reaction may refer to:* Order of reaction, in chemical kinetics, the power to which the concentration term of a certain reactant in the rate equation is raised...
with respect to the oxidant. It also assumes steady state
Steady state
A system in a steady state has numerous properties that are unchanging in time. This implies that for any property p of the system, the partial derivative with respect to time is zero:...
conditions, i.e. that transient effects do not appear.
Results
Given these assumptions, the fluxFlux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
of oxidant through each of the three phases can be expressed in terms of concentrations, material properties, and temperature. By setting the three fluxes equal to each other, they may each be found. In turn, the growth rate may be found readily from the oxidant reaction flux.
In practice, the ambient gas (stage 1) does not limit the reaction rate, so this part of the equation is often dropped. This simplification yields a simple quadratic equation for the oxide thickness. For oxide growing on an initially bare substrate, the thickness Xo at time t is given by the following equation:
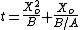
where the constants A and B encapsulate the properties of the reaction and the oxide layer, respectively.
If a wafer that already contains oxide is placed in an oxidizing ambient, this equation must be modified by adding a corrective term τ, the time that would have been required to grow the pre-existing oxide under current conditions. This term may be found using the equation for t above.
Solving the quadratic equation for Xo yields:
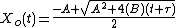
Taking the short and long time limits of the above equation reveals two main modes of operation:
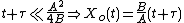
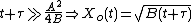
Because they appear in these equations, the quantities B and B/A are often called the quadratic and linear reaction rate constants. They depend exponentially on temperature, like this:
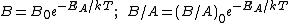
where
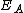 is the activation energy
is the activation energyActivation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
and
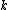 is the Boltzmann Constant in eV.
is the Boltzmann Constant in eV. 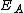 differs from one equation to the other. The following table lists the values of the four parameters for single-crystal
differs from one equation to the other. The following table lists the values of the four parameters for single-crystalCrystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
silicon under conditions typically used in industry (low doping
Doping (semiconductor)
In semiconductor production, doping intentionally introduces impurities into an extremely pure semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic...
, atmospheric
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
). The linear rate constant depends on the orientation of the crystal (usually indicated by the Miller indices of the crystal plane facing the surface). The table gives values for <100> and <111> silicon.
| Parameter | Quantity | Wet (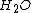 ) ) |
Dry (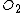 ) ) |
|---|---|---|---|
| Linear rate constant | 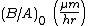 |
<100>: 9.7 <111>: 1.63 |
<100>: 3.71 <111>: 6.23 |
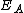 (eV (eVElectronvolt In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt... ) |
2.05 | 2.00 | |
| Parabolic rate constant | 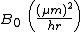 |
386 | 772 |
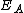 (eV) (eV) |
0.78 | 1.23 |
Validity for silicon
The Deal-Grove model works very well for single-crystal silicon under most conditions. However, experimental data shows that very thin oxides (less than about 25 nanometres) grow much more quickly in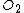 than the model predicts. This phenomenon is not well understood theoretically, but it can be modeled.
than the model predicts. This phenomenon is not well understood theoretically, but it can be modeled.If the oxide grown in a particular oxidation step will significantly exceed 25 nm, a simple adjustment accounts for the aberrant growth rate. The model yields accurate results for thick oxides if, instead of assuming zero initial thickness (or any initial thickness less than 25 nm), we assume that 25 nm of oxide exists before oxidation begins. However, for oxides near to or thinner than this threshold, more sophisticated models must be used.
Deal-Grove also fails for polycrystalline silicon ("poly-silicon"). First, the random orientation of the crystal grains makes it difficult to choose a value for the linear rate constant. Second, oxidant molecules diffuse rapidly along grain boundaries, so that poly-silicon oxidizes more rapidly than single-crystal silicon.
Dopant
Dopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
atoms strain the silicon lattice, and make it easier for silicon atoms to bond with incoming oxygen. This effect may be neglected in many cases, but heavily-doped silicon oxidizes significantly faster. The pressure of the ambient gas also affects oxidation rate.

