
Spindle checkpoint
Encyclopedia
In order to preserve one cell's identity and its proper functioning, it is necessary to maintain constant the appropriate number of chromosome
s after each cell division
. Only one error generating cells with more or less chromosomes than expected (a situation termed aneuploidy
), might lead in best cases to cell death, or alternatively it might generate catastrophic phenotypic
results:
The mechanisms verifying that all the requirements to pass to the next phase in the cell cycle
have been fulfilled are called checkpoint
s. All along the cell cycle, there are different checkpoints. The checkpoint ensuring that chromosome segregation is correct is termed spindle assembly checkpoint (SAC), spindle checkpoint or mitotic checkpoint.
During mitosis
or meiosis
, the spindle checkpoint prevents anaphase onset until all chromosome
s are properly attached to the spindle. To achieve proper segregation, the two kinetochore
s on the sister chromatid
s must be attached to opposite spindle poles (bipolar orientation). Only this pattern of attachment will ensure that each daughter cell
receives one copy of the chromosome.
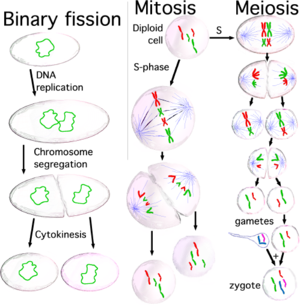 When cells are ready to divide, because cell size is big enough or because they receive the appropriate stimulus, they activate the mechanism to enter into the cell cycle, and they duplicate most organelles during S (synthesis) phase, including their centrosome. Therefore, when the cell division process will end, each daughter cell will receive a complete set of organelles. At the same time, during S phase all cells must duplicate their DNA
When cells are ready to divide, because cell size is big enough or because they receive the appropriate stimulus, they activate the mechanism to enter into the cell cycle, and they duplicate most organelles during S (synthesis) phase, including their centrosome. Therefore, when the cell division process will end, each daughter cell will receive a complete set of organelles. At the same time, during S phase all cells must duplicate their DNA
very precisely, a process termed DNA replication
. Once DNA replication has finished, in eukaryotes the DNA molecule is compacted and condensed, to form the mitotic chromosome
s, each one constituted by two sister chromatid
s, which stay hold together by the establishment of cohesion between them; each chromatid is a complete DNA molecule, attached via microtubule
s to one of the two centrosomes of the dividing cell, located at opposed poles of the cell. The structure formed by the centrosomes and the microtubules is named mitotic spindle
, due to its characteristic shape, holding the chromosomes between the two centrosomes. Both sister chromatids stay together until anaphase
; at this moment they separate from each other and they travel towards the centrosome
to which they are attached. In this way, when the two daughter cells separate at the end of the division process, each one will receive a complete set of chromatids. The mechanism responsible for the correct distribution of sister chromatids during cell division is named chromosome segregation.
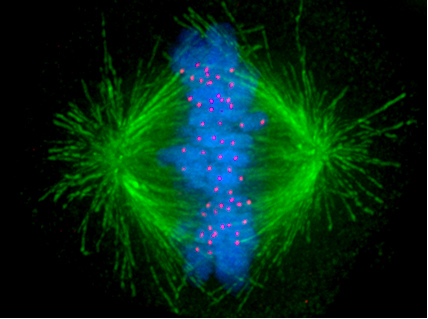
To ensure that chromosome segregation takes place correctly, cells have developed a precise and complex mechanism. In the first place, cells must coordinate centrosome
duplication with DNA replication, and a failure in this coordination will generate monopolar or multipolar mitotic spindles, which generally will produce abnormal chromosome segregation, because in this case, chromosome distribution will not take place in a balanced way.
 During S phase, the centrosome
During S phase, the centrosome
starts to duplicate. Just at the beginning of mitosis, both centriole
s achieve their maximal length, recruit additional material and their capacity to nucleate microtubules increases. As mitosis progresses, both centrosomes separate to generate the mitotic spindle. In this way, the mitotic spindle has two poles emanating microtubules. Microtubules (MTs) are long proteic filaments, with asymmetric extremities: one end termed "minus" (-) end, relatively stable and close to the centrosome, and an end termed "plus" (+) end, with alterning phases of growing-retraction, exploring the center of the cell searching the chromosomes. Each chromatid
has a special region, named the centromere
, on top of which is assembled a proteic structure termed kinetochore
, which is able to stabilize the microtubule plus end. Therefore, if by chance a microtubule exploring the center of the cell encounters a kinetochore, it may happen that the kinetochore will capture it, so that the chromosome will become attached to the spindle via the kinetochore of one of its sister chromatids. As it happens that sister chromatids are attached together and both kinetochores are located back-to-back on both chromatids, when one kinetochore becomes attached to one centrosome, the sister kinetochore becomes exposed to the centrosome located in the opposed pole; for this reason, in most cases the second kinetochore becomes associated to the centrosome in the opposed pole, via its microtubules, so that the chromosomes become « bi-oriented», a fundamental configuration (also named amphitelic) to ensure that chromosome segregation will take place correctly when the cell will divide. Occasionally, one of the two sister kinetochores may attach simultaneously to MTs generated by both poles, a configuration named merotelic, which is not detected by the spindle checkpoint but that may generate lagging chromosomes during anaphase and, consequently, aneuploidy. Merotelic orientation (characterized by the absence of tension between sister kinetochores) is frequent at the beginning of mitosis, but the protein Aurora B (a kinase conserved from yeast to vertebrates) detects and eliminates this type of anchoring. (Note: Aurora B is frequently overexpressed in various types of tumors and currently is a target for the development of anticancer drugs.)
and colchicine
, the mitotic spindle disassembles and the cell cycle is blocked at the metaphase-to-anaphase transition. Using these drugs (see the review from Rieder and Palazzo in 1992), the putative control mechanism was named Spindle Assembly Checkpoint (SAC). This regulatory mechanism has been intensively studied from then (see the review from Burke and Stukenberg in 2008).
Using different types of genetic studies, it has been established that diverse kinds of defects are able to activate de SAC: spindle depolimerization, the presence of dicentric chromosomes (with two centromeres), centromeres segregating in an aberrant way, defects in the spindle pole bodies in S. cerevisiae, defects in the kinetochore proteins, mutations in the centromeric DNA or defects in the molecular motors
active during mitosis. A summary of these observations can be found in the article from Hardwick and collaborators in 1999.
Using its own observations, Zirkle was the first to propose that “some (…) susbstance, necessary for the cell to proceed to anaphase, appears some minutes after C (moment of the arrival of the last chromosome to the metaphase plate), or after a drastic change in the cytoplasm
ic condition, just at C or immediately after C”, suggesting that this function is located on kinetochores unattached to the mitotic spindle. McIntosh extended this proposal, suggesting that one enzyme sensitive to tension located at the centromeres produces an inhibitor to the anaphase onset when the two sister kinetochores are not under bipolar tension. Indeed, the available data suggested that the signal “wait to enter in anaphase” is produced mostly on or close to unattached kinetochores. However, the primary event associated to the kinetochore attachment to the spindle, which is able to inactivate the inhibitory signal and release the metaphase arrest, could be either the acquisition of microtubules by the kinetochore (as proposed by Rieder and collaborators in 1995), or the tension stabilizing the anchoring of microtubules to the kinetochores (as suggested by the experiments realized at Nicklas' lab). Subsequent studies in cells containing two independent mitotic spindles in a sole cytoplasm
showed that the inhibitor of the metaphase-to-anaphase transition is generated by unattached kinetochores and is not freely diffusible in the cytoplasm. Yet in the same study it was shown that, once the transition from metaphase to anaphase is initiated in one part of the cell, this information is extended all along the cytoplasm
, and can overcome the signal "wait to enter in anaphase” associated to a second spindle containing unattached kinetochores.
complex and in Saccharomyces cerevisiae
is composed of at least four subunits: Smc1p, Smc3p, Scc1p (or Mcd1p) and Scc3p. Both Smc1p and Smc3p belong to the family of proteins for the Structural Maintenance of Chromosomes (SMC), which constitute a group of chromosomic ATPase
s highly conserved, and form an heterodimer (Smc1p/Smc3p). Scc1p is the homolog in S.cerevisiae of Rad21, first identified as a protein involved in DNA repair
in S. pombe. These four proteins are essential in yeast, and a mutation in any of them will produce premature sister chromatid separation. In yeast, cohesin binds to preferential sites along chromosome arms, and is very abundant close to the centromeres, as it was shown in a study using chromatin immunoprecipitation.
regions, and this suggested that the special structure or composition of heterochromatin might favour cohesin recruitment. In fact, it has been shown that Swi6 (the homolog of HP-1 in S. pombe) binds to methylated Lys
9 of histone
H3 and promotes the binding of cohesin to the centromeric repeats in S. pombe. More recent studies indicate that the RNAi
machinery regulates heterochromatin establishment, which in turn recruits cohesin to this region, both in S. pombe and in vertebrate cells. However, there must be other mechanisms than heterochromatin to ensure an augmented cohesion at centromeres, because S. cerevisiae lacks heterochromatin next to centromeres, but the presence of a functional centromere induces an increase of cohesin association in a contiguous region, spanning 20-50kb.
In this direction, Orc2 (one protein included in the origin recognition complex
, ORC, implicated in the initiation of DNA replication
during S phase
) is also located on kinetochores during mitosis in human cells; in agreement with this localization, some observations indicate that Orc2 in yeast is implicated in sister chromatid cohesion, and its removal induces SAC activation. It has also been observed that other components of the ORC complex (such as orc5 in S. pombe) are implicated in cohesion. However, the molecular pathway involving the ORC proteins seems to be additive to the cohesins' pathway, and it is mostly unknown.
(a review about this issue : Hauf and Watanabe 2004).
Indeed, a decrease in the cellular levels of cohesin generates the premature separation of sister chromatids, as well as defects in chromosome congression at the metaphase plate and delocalization of the proteins in the chromosomal passenger complex, which contains the protein Aurora B.
The proposed structure for the cohesin complex suggests that this complex connects directly both sister chromatids. In this proposed structure, the SMC components of cohesin play a structural role, so that the SMC heterodimer may function as a DNA binding protein, whose conformation is regulated by ATP
. Scc1p and Scc3p, however, would play a regulatory role.
In S. cerevisiae, Pds1p (also known as securin
) regulates sister chromatids cohesion, because it binds and inhibits the protease Esp1p (separin or separase). When anaphase onset is triggered, the anaphase-promoting complex
(APC/C or Cyclosome) degrades securin. Securin degradation releases the protease Esp1p/Separase, which degrades the cohesin rings that link the two sister chromatids, therefore promoting sister chromatids separation. It has been also shown that Polo/Cdc5 kinase
phosphorylates serine
residues next to the cutting site for Scc1, and this phosphorylation would facilitate the cutting activity.
Although this machinery is conserved through evolution, in vertebrates most cohesin molecules are released in prophase, independently of the presence of the APC/C, in a process dependent on Polo-like 1 (PLK1
) and Aurora B. Yet it has been shown that a small quantity of Scc1 remains associated to centromeres in human cells until metaphase, and a similar amount is cut in anaphase, when it disappears from centromeres. On the other hand, some experiments show that sister chromatids cohesion in the arms is lost gradually after sister centromeres have separated, and sister chromatids move toward the opposite poles of the cell.
According to some observations, a fraction of cohesins in the chromosomal arms and the centromeric cohesins are protected by the protein Shugoshin (Sgo1), avoiding their release during prophase. To be able to function as protector for the centromeric cohesion, Sgo1 must be inactivated at the beginning of anaphase, as well as Pds1p. In fact, both Pds1p and Sgo1 are substrates of APC/C in vertebrates.
At the metaphase to anaphase transition, this cohesion between sister chromatids is dissolved, and the separated chromatids are pulled to opposite sides of the cell by the spindle microtubules. The chromatids are further separated by the physical movement of the spindle poles themselves. Premature dissociation of the chromatids can lead to chromosome missegregation and aneuploidy in the daughter cells. Thus, the job of the metaphase checkpoint is to prevent this transition into anaphase until the chromosomes are properly attached, before the sister chromatids separate.
/MAD3 (mitotic arrest deficient), BUB3
(budding uninhibited by benzimidazole), and CDC20
. Other proteins involved in the SAC include MAD1
, BUB1
, MPS1, and Aurora B
. For higher eukaryotes, additional regulators of the SAC include constituents of the ROD-ZW10 complex
, p31comet, MAPK
, CDK1-cyclin-B
, NEK2
, and PLK1
.
, CDC20 and the SAC proteins concentrate at the kinetochores before attachment to the spindle assembly. These proteins keep the SAC activated until they are removed and the correct kinetochore-microtubule attachment is made. Even a single unattached kinetochore can maintain the spindle checkpoint. After attachment of microtubule plus-ends and formation of kinetochore microtubules, MAD1 and MAD2 are depleted from the kinetochore assembly. Another regulator of checkpoint activation is kinetochore tension. When sister kinetochores are properly attached to opposite spindle poles, forces in the mitotic spindle generate tension at the kinetochores. Bi-oriented sister kinetochores stabilize the kinetochore-microtubule assembly whereas weak tension has a destabilizing effect. In response to incorrect kinetochore attachments such as syntelic attachment, where both kinetochores becomes attached to one spindle pole, the weak tension generated destabilizes the incorrect attachment and allows the kinetochore to reattach correctly to the spindle body. During this process, kinetochores that are attached to the mitotic spindle but that are not under tension trigger the spindle checkpoint. Aurora-B/Ipl1 kinase of the chromosomal passenger complex functions as the tensions sensor in improper kinetochore attachments. It detects and destabilizes incorrect attachments through control of the microtubule-severing KINI kinesin MCAK, the DASH complex, and the Ndc80/Hec1
complex at the microtubule-kinetochore interface. The Aurora-B/Ipl1 kinase is also critical in correcting merotelic attachments, where one kinetochore is simultaneously attached to both spindle poles. Merotelic attachments generate sufficient tension and are not detected by the SAC, and without correction, may result in chromosome mis-segregation due to slow chromatid migration speed. While microtubule attachment is independently required for SAC activation, it is unclear whether tension is an independent regulator of SAC, although it is clear that differing regulatory behaviors arise with tension.
Once activated, the spindle checkpoint blocks anaphase
entry by inhibiting the anaphase-promoting complex
via regulation of the activity of mitotic checkpoint complex. The mechanism of inhibition of APC by the mitotic checkpoint complex is poorly understood, although it is hypothesized that the MCC binds to APC as a pseudosubstrate using the KEN-box motif in BUBR1. At the same time that mitotic checkpoint complex is being activated, the centromere
protein CENP-E activates BUBR1, which also blocks anaphase.
together with MAD2 and MAD3 bound to Cdc20
. MAD2 and MAD3 have distinct binding sites on CDC20, and act synergistically to inhibit APC/C. The MAD3 complex is composed of BUB3, which binds to Mad3 and BUB1B
through the short linear motif
known as the GLEBS motif. The exact order of attachments which must take place in order to form the MCC remains unknown. It is possible that Mad2-Cdc20 form a complex at the same time as BUBR1-BUB3-Cdc20 form another complex, and these two subcomplexes are consequently combined to form the mitotic checkpoint complex. In human cells, binding of BUBR1 to CDC20 requires prior binding of MAD2 to CDC20, so it is possible that the MAD2-CDC20 subcomplex acts as an initiator for MCC formation. BUBR1 depletion leads only to a mild reduction in Mad2-Cdc20 levels while Mad2 is required for the binding of BubR1-Bub3 to Cdc20. Nevertheless BUBR1 is still required for checkpoint activation.
The mechanism of formation for the MCC is unclear and there are competing theories for both kinetochore-dependent and kinetochore-independent formation. In support of the kinetochore-independent theory, MCC is detectable in S. cerevisiae cells in which core kinetocore assembly proteins have been mutated and cells in which the SAC has been deactivated, which suggests that the MCC could be assembled during mitosis without kinetochore localization. In one model, unattached prometaphase kinetochores can 'sensitize' APC to inhibition of MCC by recruiting the APC to kinetochores via a functioning SAC. Furthermore, depletions of various SAC proteins have revealed that MAD2 and BUBR1 depletions affect the timing of mitosis independently of kinetochores, while depletions of other SAC proteins result in a dysfunctional SAC without altering the duration of mitosis. Thus it is possible that the SAC functions through a two-stage timer where MAD2 and BUBR1 control the duration of mitosis in the first stage, which may be extended in the second stage if there are unattached kinetochores as well as other SAC proteins. However, there are lines of evidence which are in disfavor of the kinetochore-independent assembly. MCC has yet to be found during interphase
, while MCC does not form from its constituents in X. laevis meiosis II extracts without the addition of sperm of nuclei and nocodazole
to prevent spindle assembly.
The leading model of MCC formation is the "MAD2-template model", which depends on the kinetochore dynamics of MAD2 to create the MCC. MAD1 localizes to unattached kinetochores while binding strongly to MAD2. The localization of MAD2 and BubR1 to the kinetochore may also be dependent on the Aurora B kinase
. Cells lacking Aurora B fail to arrest in metaphase even when chromosomes lack microtubule attachment. Unattached kinetochores first bind to a MAD1-C-MAD2-p31comet complex and releases the p31comet through unknown mechanisms. The resulting MAD-C-MAD2 complex recruits the open conformer of Mad2 (O-Mad2) to the kinetochores. This O-Mad2 changes its conformation to closed Mad2 (C-Mad2) and binds Mad1. This Mad1/C-Mad2 complex is responsible for the recruitment of more O-Mad2 to the kinetochores, which changes its conformation to C-Mad2 and binds Cdc20 in an auto-amplification reaction. Since MAD1 and CDC20 both contain a similar MAD2-binding motif, the empty O-MAD2 conformation changes to C-MAD2 while binding to CDC20. This positive feedback loop is negatively regulated by p31comet, which competitively binds to C-MAD2 bound to either MAD1 or CDC20 and reduces further O-MAD2 binding to C-MAD2. Further control mechanisms may also exist, considering that p31comet is not present in lower eukaryotes. The 'template model' nomenclature is thus derived from the process where MAD1-C-MAD2 acts as a template for the formation of C-MAD2-CDC20 copies. This sequestration of Cdc20 is essential for maintaining the spindle checkpoint.
. Upon microtubule-kinetochore attachment, a mechanism of stripping via a dynein-dynein motor complex
transports spindle checkpoint proteins away from the kinetochores. The stripped proteins, which include MAD1, MAD2, MPS1, and CENP-F
, are then redistributed to the spindle poles. The stripping process is highly dependent on undamaged microtubule structure as well as dynein motility along microtubules. As well as functioning as a regulator of the C-MAD2 positive feedback loop, p31comet also may act as a decativator of the SAC. Unattached kinetochores temporarily inactivate p31comet, but attachment reactivates the protein and inhibits MAD2 activation, possibly by inhibitory phosphorylation. Another possible mechanism of SAC inactivation results from energy-dependent dissociation of the MAD2-CDC20 complex through non-degradative ubiquitylation of CDC20. Conversely, the de-ubiquitylating enzyme protectin is required to maintain the SAC. Thus, unattached kinetochores maintain the checkpoint by continuously recreating the MAD2-CDC20 subcomplex from its components. The SAC may also be deativated by APC activation induced proteolysis
. Since the SAC is not reactivated by the loss of sister-chromatid cohesion during anaphase, the proteolysis of cyclin B and inactivation of the CDK1-cyclin-B kinase also inhibits SAC activity. Degradation of MPS1 during anaphase prevents the reactivation of SAC after removal of sister-chromatid cohesion. After checkpoint deactivation and during the normal anaphase of the cell cycle, the anaphase promoting complex is activated through decreasing MCC activity. When this happens the enzyme complex polyubiquitinates the anaphase inhibitor securin
. The ubiquitination and destruction of securin at the end of metaphase releases the active protease called separase. Separase cleaves the cohesion molecules that hold the sister chromatids together to activate anaphase.
and even tumorigenesis. Due to the fact that alterations in mitotic regulatory proteins can lead to aneuploidy and this is a frequent event in cancer
, it was initially thought that these genes could be mutated in cancerous tissues. Subsequent studies in different laboratories have not found a higher frequency of mutations in these genes, although the spindle checkpoint is not working properly in many cases. What it has do been detected is that variations in the physiological levels of these proteins (such as Mad2 or BubR1) are associated with aneuploidy and tumorigenesis, and this has been demonstrated using animal model
s.
However, recent studies indicate that what seems to happen is a more complicated scenario: aneuploidy would drive a high incidence of tumorigenesis only when alterations in the levels of specific mitotic checkpoint components (either reduction or overexpression) in tissues is also inducing other defects able to predispose them to tumors.
That is, defects such as an increase in DNA damage, chromosomal rearrangements, and/or a decreased incidence of cell death. For some mitotic checkpoint components, it is known that they are implicated in functions outside mitosis: nuclear import (Mad1), transcriptional repression (Bub3), and cell death, DNA damage response, aging, and megakaryopoiesis for BubR1. All this supports the conclusion that increase in tumorigenesis is associated with defects other than aneuploidy alone.
Chromosome
A chromosome is an organized structure of DNA and protein found in cells. It is a single piece of coiled DNA containing many genes, regulatory elements and other nucleotide sequences. Chromosomes also contain DNA-bound proteins, which serve to package the DNA and control its functions.Chromosomes...
s after each cell division
Cell division
Cell division is the process by which a parent cell divides into two or more daughter cells . Cell division is usually a small segment of a larger cell cycle. This type of cell division in eukaryotes is known as mitosis, and leaves the daughter cell capable of dividing again. The corresponding sort...
. Only one error generating cells with more or less chromosomes than expected (a situation termed aneuploidy
Aneuploidy
Aneuploidy is an abnormal number of chromosomes, and is a type of chromosome abnormality. An extra or missing chromosome is a common cause of genetic disorders . Some cancer cells also have abnormal numbers of chromosomes. Aneuploidy occurs during cell division when the chromosomes do not separate...
), might lead in best cases to cell death, or alternatively it might generate catastrophic phenotypic
Phenotype
A phenotype is an organism's observable characteristics or traits: such as its morphology, development, biochemical or physiological properties, behavior, and products of behavior...
results:
- In humans, Down syndromeDown syndromeDown syndrome, or Down's syndrome, trisomy 21, is a chromosomal condition caused by the presence of all or part of an extra 21st chromosome. It is named after John Langdon Down, the British physician who described the syndrome in 1866. The condition was clinically described earlier in the 19th...
appears in children carrying in their cells one extra copy of chromosome 21, as a result of a defect in chromosome segregationChromosome segregationChromosome segregation is a step in cell reproduction or division, where chromosomes pair off with their similar homologous chromosome. In mitosis, a complete copy of each one is made. In meiosis, one chromosome from each pair migrates to opposite ends of the cell and the genes are split to make a...
during meiosisMeiosisMeiosis is a special type of cell division necessary for sexual reproduction. The cells produced by meiosis are gametes or spores. The animals' gametes are called sperm and egg cells....
in one of the progenitors. This defect will generate a gameteGameteA gamete is a cell that fuses with another cell during fertilization in organisms that reproduce sexually...
(spermatozoide or oocyte) with an extra chromosome 21. After fecundation, this gamete will generate an embryoEmbryoAn embryo is a multicellular diploid eukaryote in its earliest stage of development, from the time of first cell division until birth, hatching, or germination...
with three copies of chromosome 21. - In cancer cells, aneuploidyAneuploidyAneuploidy is an abnormal number of chromosomes, and is a type of chromosome abnormality. An extra or missing chromosome is a common cause of genetic disorders . Some cancer cells also have abnormal numbers of chromosomes. Aneuploidy occurs during cell division when the chromosomes do not separate...
is a frequent event, indicating that these cells present a defect in the machinery involved in chromosome segregationChromosome segregationChromosome segregation is a step in cell reproduction or division, where chromosomes pair off with their similar homologous chromosome. In mitosis, a complete copy of each one is made. In meiosis, one chromosome from each pair migrates to opposite ends of the cell and the genes are split to make a...
, as well as in the mechanism ensuring that segregation is correctly performed.
The mechanisms verifying that all the requirements to pass to the next phase in the cell cycle
Cell cycle
The cell cycle, or cell-division cycle, is the series of events that takes place in a cell leading to its division and duplication . In cells without a nucleus , the cell cycle occurs via a process termed binary fission...
have been fulfilled are called checkpoint
Checkpoint
- Places :* Border checkpoint, a place on the land border between two states where travellers and/or goods are inspected** Checkpoint Charlie, the best known crossing point between East Berlin and West Berlin during the Cold War...
s. All along the cell cycle, there are different checkpoints. The checkpoint ensuring that chromosome segregation is correct is termed spindle assembly checkpoint (SAC), spindle checkpoint or mitotic checkpoint.
During mitosis
Mitosis
Mitosis is the process by which a eukaryotic cell separates the chromosomes in its cell nucleus into two identical sets, in two separate nuclei. It is generally followed immediately by cytokinesis, which divides the nuclei, cytoplasm, organelles and cell membrane into two cells containing roughly...
or meiosis
Meiosis
Meiosis is a special type of cell division necessary for sexual reproduction. The cells produced by meiosis are gametes or spores. The animals' gametes are called sperm and egg cells....
, the spindle checkpoint prevents anaphase onset until all chromosome
Chromosome
A chromosome is an organized structure of DNA and protein found in cells. It is a single piece of coiled DNA containing many genes, regulatory elements and other nucleotide sequences. Chromosomes also contain DNA-bound proteins, which serve to package the DNA and control its functions.Chromosomes...
s are properly attached to the spindle. To achieve proper segregation, the two kinetochore
Kinetochore
The kinetochore is the protein structure on chromatids where the spindle fibers attach during cell division to pull sister chromatids apart....
s on the sister chromatid
Chromatid
A chromatid is one of the two identical copies of DNA making up a duplicated chromosome, which are joined at their centromeres, for the process of cell division . They are called sister chromatids so long as they are joined by the centromeres...
s must be attached to opposite spindle poles (bipolar orientation). Only this pattern of attachment will ensure that each daughter cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
receives one copy of the chromosome.
Cell division: duplication of material and distribution to daughter cells

DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
very precisely, a process termed DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
. Once DNA replication has finished, in eukaryotes the DNA molecule is compacted and condensed, to form the mitotic chromosome
Chromosome
A chromosome is an organized structure of DNA and protein found in cells. It is a single piece of coiled DNA containing many genes, regulatory elements and other nucleotide sequences. Chromosomes also contain DNA-bound proteins, which serve to package the DNA and control its functions.Chromosomes...
s, each one constituted by two sister chromatid
Chromatid
A chromatid is one of the two identical copies of DNA making up a duplicated chromosome, which are joined at their centromeres, for the process of cell division . They are called sister chromatids so long as they are joined by the centromeres...
s, which stay hold together by the establishment of cohesion between them; each chromatid is a complete DNA molecule, attached via microtubule
Microtubule
Microtubules are a component of the cytoskeleton. These rope-like polymers of tubulin can grow as long as 25 micrometers and are highly dynamic. The outer diameter of microtubule is about 25 nm. Microtubules are important for maintaining cell structure, providing platforms for intracellular...
s to one of the two centrosomes of the dividing cell, located at opposed poles of the cell. The structure formed by the centrosomes and the microtubules is named mitotic spindle
Mitotic spindle
In cell biology, the spindle fibers are the structure that separates the chromosomes into the daughter cells during cell division. It is part of the cytoskeleton in eukaryotic cells...
, due to its characteristic shape, holding the chromosomes between the two centrosomes. Both sister chromatids stay together until anaphase
Anaphase
Anaphase, from the ancient Greek ἀνά and φάσις , is the stage of mitosis or meiosis when chromosomes move to opposite poles of the cell....
; at this moment they separate from each other and they travel towards the centrosome
Centrosome
In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center of the animal cell as well as a regulator of cell-cycle progression. It was discovered by Edouard Van Beneden in 1883...
to which they are attached. In this way, when the two daughter cells separate at the end of the division process, each one will receive a complete set of chromatids. The mechanism responsible for the correct distribution of sister chromatids during cell division is named chromosome segregation.
To ensure that chromosome segregation takes place correctly, cells have developed a precise and complex mechanism. In the first place, cells must coordinate centrosome
Centrosome
In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center of the animal cell as well as a regulator of cell-cycle progression. It was discovered by Edouard Van Beneden in 1883...
duplication with DNA replication, and a failure in this coordination will generate monopolar or multipolar mitotic spindles, which generally will produce abnormal chromosome segregation, because in this case, chromosome distribution will not take place in a balanced way.
Mitosis: anchoring of chromosomes to the spindle and chromosome segregation

Centrosome
In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center of the animal cell as well as a regulator of cell-cycle progression. It was discovered by Edouard Van Beneden in 1883...
starts to duplicate. Just at the beginning of mitosis, both centriole
Centriole
A Centriole is a barrel-shaped cell structure found in most animal eukaryotic cells, though it is absent in higher plants and most fungi. The walls of each centriole are usually composed of nine triplets of microtubules...
s achieve their maximal length, recruit additional material and their capacity to nucleate microtubules increases. As mitosis progresses, both centrosomes separate to generate the mitotic spindle. In this way, the mitotic spindle has two poles emanating microtubules. Microtubules (MTs) are long proteic filaments, with asymmetric extremities: one end termed "minus" (-) end, relatively stable and close to the centrosome, and an end termed "plus" (+) end, with alterning phases of growing-retraction, exploring the center of the cell searching the chromosomes. Each chromatid
Chromatid
A chromatid is one of the two identical copies of DNA making up a duplicated chromosome, which are joined at their centromeres, for the process of cell division . They are called sister chromatids so long as they are joined by the centromeres...
has a special region, named the centromere
Centromere
A centromere is a region of DNA typically found near the middle of a chromosome where two identical sister chromatids come closest in contact. It is involved in cell division as the point of mitotic spindle attachment...
, on top of which is assembled a proteic structure termed kinetochore
Kinetochore
The kinetochore is the protein structure on chromatids where the spindle fibers attach during cell division to pull sister chromatids apart....
, which is able to stabilize the microtubule plus end. Therefore, if by chance a microtubule exploring the center of the cell encounters a kinetochore, it may happen that the kinetochore will capture it, so that the chromosome will become attached to the spindle via the kinetochore of one of its sister chromatids. As it happens that sister chromatids are attached together and both kinetochores are located back-to-back on both chromatids, when one kinetochore becomes attached to one centrosome, the sister kinetochore becomes exposed to the centrosome located in the opposed pole; for this reason, in most cases the second kinetochore becomes associated to the centrosome in the opposed pole, via its microtubules, so that the chromosomes become « bi-oriented», a fundamental configuration (also named amphitelic) to ensure that chromosome segregation will take place correctly when the cell will divide. Occasionally, one of the two sister kinetochores may attach simultaneously to MTs generated by both poles, a configuration named merotelic, which is not detected by the spindle checkpoint but that may generate lagging chromosomes during anaphase and, consequently, aneuploidy. Merotelic orientation (characterized by the absence of tension between sister kinetochores) is frequent at the beginning of mitosis, but the protein Aurora B (a kinase conserved from yeast to vertebrates) detects and eliminates this type of anchoring. (Note: Aurora B is frequently overexpressed in various types of tumors and currently is a target for the development of anticancer drugs.)
Discovery of the Spindle Assembly Checkpoint (SAC)
Zirkle (in 1970) was one of the first researchers to observe that, when just one chromosome is retarded to arrive at the metaphase plate, anaphase onset is postponed until some minutes after its arrival. This observation, together with similar ones, suggested that it exists a control mechanism at the metaphase-to-anaphase transition. Using drugs such as nocodazoleNocodazole
Nocodazole is an anti-neoplastic agent which exerts its effect in cells by interfering with the polymerization of microtubules. Microtubules are one type of fibre which constitutes the cytoskeleton, and the dynamic microtubule network has several important roles in the cell, including vesicular...
and colchicine
Colchicine
Colchicine is a medication used for gout. It is a toxic natural product and secondary metabolite, originally extracted from plants of the genus Colchicum...
, the mitotic spindle disassembles and the cell cycle is blocked at the metaphase-to-anaphase transition. Using these drugs (see the review from Rieder and Palazzo in 1992), the putative control mechanism was named Spindle Assembly Checkpoint (SAC). This regulatory mechanism has been intensively studied from then (see the review from Burke and Stukenberg in 2008).
Using different types of genetic studies, it has been established that diverse kinds of defects are able to activate de SAC: spindle depolimerization, the presence of dicentric chromosomes (with two centromeres), centromeres segregating in an aberrant way, defects in the spindle pole bodies in S. cerevisiae, defects in the kinetochore proteins, mutations in the centromeric DNA or defects in the molecular motors
Molecular motors
Molecular motors are biological molecular machines that are the essential agents of movement in living organisms. Generally speaking, a motor may be defined as a device that consumes energy in one form and converts it into motion or mechanical work; for example, many protein-based molecular motors...
active during mitosis. A summary of these observations can be found in the article from Hardwick and collaborators in 1999.
Using its own observations, Zirkle was the first to propose that “some (…) susbstance, necessary for the cell to proceed to anaphase, appears some minutes after C (moment of the arrival of the last chromosome to the metaphase plate), or after a drastic change in the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
ic condition, just at C or immediately after C”, suggesting that this function is located on kinetochores unattached to the mitotic spindle. McIntosh extended this proposal, suggesting that one enzyme sensitive to tension located at the centromeres produces an inhibitor to the anaphase onset when the two sister kinetochores are not under bipolar tension. Indeed, the available data suggested that the signal “wait to enter in anaphase” is produced mostly on or close to unattached kinetochores. However, the primary event associated to the kinetochore attachment to the spindle, which is able to inactivate the inhibitory signal and release the metaphase arrest, could be either the acquisition of microtubules by the kinetochore (as proposed by Rieder and collaborators in 1995), or the tension stabilizing the anchoring of microtubules to the kinetochores (as suggested by the experiments realized at Nicklas' lab). Subsequent studies in cells containing two independent mitotic spindles in a sole cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
showed that the inhibitor of the metaphase-to-anaphase transition is generated by unattached kinetochores and is not freely diffusible in the cytoplasm. Yet in the same study it was shown that, once the transition from metaphase to anaphase is initiated in one part of the cell, this information is extended all along the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
, and can overcome the signal "wait to enter in anaphase” associated to a second spindle containing unattached kinetochores.
Cohesin: SMC proteins
As it has been previously noted, sister chromatids stay associated from S phase (when DNA is replicated to generate two identic copies, the two chromatids) until anaphase. At this point, the two sister chromatids separate and travel to opposite poles in the dividing cell. Genetic and biochemical studies in yeast and in egg's extracts in Xenopus laevis identified a polyprotein complex as an essential player in sister chromatids cohesion (see the review from Hirano in 2000). This complex is known as the cohesinCohesin
Cohesin is a protein complex that regulates the separation of sister chromatids during cell division, either mitosis or meiosis.- Structure :...
complex and in Saccharomyces cerevisiae
Saccharomyces cerevisiae
Saccharomyces cerevisiae is a species of yeast. It is perhaps the most useful yeast, having been instrumental to baking and brewing since ancient times. It is believed that it was originally isolated from the skin of grapes...
is composed of at least four subunits: Smc1p, Smc3p, Scc1p (or Mcd1p) and Scc3p. Both Smc1p and Smc3p belong to the family of proteins for the Structural Maintenance of Chromosomes (SMC), which constitute a group of chromosomic ATPase
ATPase
ATPases are a class of enzymes that catalyze the decomposition of adenosine triphosphate into adenosine diphosphate and a free phosphate ion. This dephosphorylation reaction releases energy, which the enzyme harnesses to drive other chemical reactions that would not otherwise occur...
s highly conserved, and form an heterodimer (Smc1p/Smc3p). Scc1p is the homolog in S.cerevisiae of Rad21, first identified as a protein involved in DNA repair
DNA repair
DNA repair refers to a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as UV light and radiation can cause DNA damage, resulting in as many as 1...
in S. pombe. These four proteins are essential in yeast, and a mutation in any of them will produce premature sister chromatid separation. In yeast, cohesin binds to preferential sites along chromosome arms, and is very abundant close to the centromeres, as it was shown in a study using chromatin immunoprecipitation.
The role of heterochromatin
Classical cytologic observations suggested that sister chromatids are more strongly attached at heterochromaticHeterochromatin
Heterochromatin is a tightly packed form of DNA, which comes in different varieties. These varieties lie on a continuum between the two extremes of constitutive and facultative heterochromatin...
regions, and this suggested that the special structure or composition of heterochromatin might favour cohesin recruitment. In fact, it has been shown that Swi6 (the homolog of HP-1 in S. pombe) binds to methylated Lys
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
9 of histone
Histone
In biology, histones are highly alkaline proteins found in eukaryotic cell nuclei that package and order the DNA into structural units called nucleosomes. They are the chief protein components of chromatin, acting as spools around which DNA winds, and play a role in gene regulation...
H3 and promotes the binding of cohesin to the centromeric repeats in S. pombe. More recent studies indicate that the RNAi
RNAI
RNAI is a non-coding RNA that is an antisense repressor of the replication of some E. coli plasmids, including ColE1. Plasmid replication is usually initiated by RNAII, which acts as a primer by binding to its template DNA. The complementary RNAI binds RNAII prohibiting it from its initiation role...
machinery regulates heterochromatin establishment, which in turn recruits cohesin to this region, both in S. pombe and in vertebrate cells. However, there must be other mechanisms than heterochromatin to ensure an augmented cohesion at centromeres, because S. cerevisiae lacks heterochromatin next to centromeres, but the presence of a functional centromere induces an increase of cohesin association in a contiguous region, spanning 20-50kb.
In this direction, Orc2 (one protein included in the origin recognition complex
Origin Recognition Complex
ORC or Origin Recognition Complex is a multi-subunit DNA binding complex that binds in all eukaryotes in an ATP-dependent manner to origins of replication....
, ORC, implicated in the initiation of DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
during S phase
S phase
S-phase is the part of the cell cycle in which DNA is replicated, occurring between G1 phase and G2 phase. Precise and accurate DNA replication is necessary to prevent genetic abnormalities which often lead to cell death or disease. Due to the importance, the regulatory pathways that govern this...
) is also located on kinetochores during mitosis in human cells; in agreement with this localization, some observations indicate that Orc2 in yeast is implicated in sister chromatid cohesion, and its removal induces SAC activation. It has also been observed that other components of the ORC complex (such as orc5 in S. pombe) are implicated in cohesion. However, the molecular pathway involving the ORC proteins seems to be additive to the cohesins' pathway, and it is mostly unknown.
Function of cohesion and its disolution
Centromeric cohesion resists the forces exerted by spindle microtubules towards the poles, which generate tension between sister kinetochores. In turn, this tension stabilizes the attachment microtubule-kinetochore, through a mechanism implicating the protein Aurora BAurora B kinase
Aurora B kinase is a protein that functions in the attachment of the mitotic spindle to the centromere.In cancerous cells, over-expression of these enzymes causes unequal distribution of genetic information, creating aneuploid cells, a hallmark of cancer....
(a review about this issue : Hauf and Watanabe 2004).
Indeed, a decrease in the cellular levels of cohesin generates the premature separation of sister chromatids, as well as defects in chromosome congression at the metaphase plate and delocalization of the proteins in the chromosomal passenger complex, which contains the protein Aurora B.
The proposed structure for the cohesin complex suggests that this complex connects directly both sister chromatids. In this proposed structure, the SMC components of cohesin play a structural role, so that the SMC heterodimer may function as a DNA binding protein, whose conformation is regulated by ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
. Scc1p and Scc3p, however, would play a regulatory role.
In S. cerevisiae, Pds1p (also known as securin
Securin
Securin is a protein involved in control of the metaphase-anaphase transition and anaphase onset. Following bi-orientation of chromosome pairs and inactivation of the spindle checkpoint system, the underlying regulatory system, which includes securin, produces an abrupt stimulus that induces highly...
) regulates sister chromatids cohesion, because it binds and inhibits the protease Esp1p (separin or separase). When anaphase onset is triggered, the anaphase-promoting complex
Anaphase-promoting complex
Anaphase-Promoting Complex, also called cyclosome , is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin and RING subunit much like SCF...
(APC/C or Cyclosome) degrades securin. Securin degradation releases the protease Esp1p/Separase, which degrades the cohesin rings that link the two sister chromatids, therefore promoting sister chromatids separation. It has been also shown that Polo/Cdc5 kinase
Kinase
In chemistry and biochemistry, a kinase is a type of enzyme that transfers phosphate groups from high-energy donor molecules, such as ATP, to specific substrates, a process referred to as phosphorylation. Kinases are part of the larger family of phosphotransferases...
phosphorylates serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
residues next to the cutting site for Scc1, and this phosphorylation would facilitate the cutting activity.
Although this machinery is conserved through evolution, in vertebrates most cohesin molecules are released in prophase, independently of the presence of the APC/C, in a process dependent on Polo-like 1 (PLK1
PLK1
Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 or serine/threonine-protein kinase 13 , is an enzyme that in humans is encoded by the PLK1 gene.- Structure :...
) and Aurora B. Yet it has been shown that a small quantity of Scc1 remains associated to centromeres in human cells until metaphase, and a similar amount is cut in anaphase, when it disappears from centromeres. On the other hand, some experiments show that sister chromatids cohesion in the arms is lost gradually after sister centromeres have separated, and sister chromatids move toward the opposite poles of the cell.
According to some observations, a fraction of cohesins in the chromosomal arms and the centromeric cohesins are protected by the protein Shugoshin (Sgo1), avoiding their release during prophase. To be able to function as protector for the centromeric cohesion, Sgo1 must be inactivated at the beginning of anaphase, as well as Pds1p. In fact, both Pds1p and Sgo1 are substrates of APC/C in vertebrates.
Metaphase to Anaphase Transition
The beginning of metaphase is characterized by the connection of the microtubules to the kinetochores of the chromosomes, as well as the alignment of the chromosomes in the middle of the cell. Each chromatid has its own kinetochore, and all of the microtubules that are bound to kinetochores of sister chromatids radiate from opposite poles of the cell. These microtubules exert a pulling force on the chromosomes towards the opposite ends of the cells, while the cohesion between the sister chromatids oppose this force.At the metaphase to anaphase transition, this cohesion between sister chromatids is dissolved, and the separated chromatids are pulled to opposite sides of the cell by the spindle microtubules. The chromatids are further separated by the physical movement of the spindle poles themselves. Premature dissociation of the chromatids can lead to chromosome missegregation and aneuploidy in the daughter cells. Thus, the job of the metaphase checkpoint is to prevent this transition into anaphase until the chromosomes are properly attached, before the sister chromatids separate.
Spindle Assembly Checkpoint Overview
The spindle assembly checkpoint (SAC) is an active signal produced by improperly attached kinetochores, which is conserved in all eukaryotes. The SAC stops the cell cycle by negatively regulating CDC20, thereby preventing the activation of the polyubiquitylation activities of anaphase promoting complex (APC). The proteins responsible for the SAC signal compose the mitotic checkpoint complex (MCC), which includes SAC proteins, MAD2MAD2
MAD2 is an essential spindle checkpoint protein. The spindle checkpoint system is a regulatory system that restrains progression through the metaphase-to-anaphase transition. The Mad2 gene was first identified in the yeast S. cerevisiae in a screen for genes which when mutated would confer...
/MAD3 (mitotic arrest deficient), BUB3
BUB3
Mitotic checkpoint protein BUB3 is a protein that in humans is encoded by the BUB3 gene.Bub3 is a protein involved with the regulation of the Spindle Assembly Checkpoint ; though BUB3 is non-essential in yeast, it is essential in higher eukaryotes...
(budding uninhibited by benzimidazole), and CDC20
CDC20
The cell-division cycle protein 20 is an essential regulator of cell division that is encoded by the CDC20 gene in humans. To the best of current knowledge its most important function is to activate the anaphase promoting complex , a large 11-13 subunit complex that initiates chromatid separation...
. Other proteins involved in the SAC include MAD1
MAD1
Mad1 is a non-essential protein in yeast which has a function in the spindle assembly checkpoint .This checkpoint monitors chromosome attachment to spindle microtubules and prevents cells from starting anaphase until the spindle is built up. The name Mad refers to the observation that mutant cells...
, BUB1
BUB1
Mitotic checkpoint serine/threonine-protein kinase BUB1 also known as BUB1 is an enzyme that in humans is encoded by the BUB1 gene....
, MPS1, and Aurora B
Aurora B kinase
Aurora B kinase is a protein that functions in the attachment of the mitotic spindle to the centromere.In cancerous cells, over-expression of these enzymes causes unequal distribution of genetic information, creating aneuploid cells, a hallmark of cancer....
. For higher eukaryotes, additional regulators of the SAC include constituents of the ROD-ZW10 complex
ZW10
Centromere/kinetochore protein zw10 homolog is a protein that in humans is encoded by the ZW10 gene.-Further reading:...
, p31comet, MAPK
Kinesin
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule filaments, and are powered by the hydrolysis of ATP . The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular...
, CDK1-cyclin-B
Cdk1
Cyclin dependent kinase 1 also known as Cdk1 or cell division control protein 2 homolog is a highly conserved protein that functions as a serine/threonine kinase, and is a key player in cell cycle regulation. It has been highly studied in the budding yeast S. cerevisiae, and the fission yeast S....
, NEK2
NEK2
Serine/threonine-protein kinase Nek2 is an enzyme that in humans is encoded by the NEK2 gene.-Interactions:NEK2 has been shown to interact with MAPK1 and NDC80.-Further reading:...
, and PLK1
PLK1
Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 or serine/threonine-protein kinase 13 , is an enzyme that in humans is encoded by the PLK1 gene.- Structure :...
.
Checkpoint activation
The SAC monitors the interaction between improperly connected kinetochores and spindle microtubules, and is maintained until kinetochores are properly attached to the spindle. During prometaphasePrometaphase
Prometaphase is the phase of mitosis following prophase and preceding metaphase, in eukaryotic somatic cells. In Prometaphase, The nuclear envelope breaks into fragments and disappears. The tiny nucleolus inside the nuclear envolope, also dissolves. Microtubules emerging from the centrosomes at the...
, CDC20 and the SAC proteins concentrate at the kinetochores before attachment to the spindle assembly. These proteins keep the SAC activated until they are removed and the correct kinetochore-microtubule attachment is made. Even a single unattached kinetochore can maintain the spindle checkpoint. After attachment of microtubule plus-ends and formation of kinetochore microtubules, MAD1 and MAD2 are depleted from the kinetochore assembly. Another regulator of checkpoint activation is kinetochore tension. When sister kinetochores are properly attached to opposite spindle poles, forces in the mitotic spindle generate tension at the kinetochores. Bi-oriented sister kinetochores stabilize the kinetochore-microtubule assembly whereas weak tension has a destabilizing effect. In response to incorrect kinetochore attachments such as syntelic attachment, where both kinetochores becomes attached to one spindle pole, the weak tension generated destabilizes the incorrect attachment and allows the kinetochore to reattach correctly to the spindle body. During this process, kinetochores that are attached to the mitotic spindle but that are not under tension trigger the spindle checkpoint. Aurora-B/Ipl1 kinase of the chromosomal passenger complex functions as the tensions sensor in improper kinetochore attachments. It detects and destabilizes incorrect attachments through control of the microtubule-severing KINI kinesin MCAK, the DASH complex, and the Ndc80/Hec1
NDC80
Kinetochore protein NDC80 homolog is a protein that in humans is encoded by the NDC80 gene.-Interactions:NDC80 has been shown to interact with MIS12, NEK2 and PSMC2.-Further reading:...
complex at the microtubule-kinetochore interface. The Aurora-B/Ipl1 kinase is also critical in correcting merotelic attachments, where one kinetochore is simultaneously attached to both spindle poles. Merotelic attachments generate sufficient tension and are not detected by the SAC, and without correction, may result in chromosome mis-segregation due to slow chromatid migration speed. While microtubule attachment is independently required for SAC activation, it is unclear whether tension is an independent regulator of SAC, although it is clear that differing regulatory behaviors arise with tension.
Once activated, the spindle checkpoint blocks anaphase
Anaphase
Anaphase, from the ancient Greek ἀνά and φάσις , is the stage of mitosis or meiosis when chromosomes move to opposite poles of the cell....
entry by inhibiting the anaphase-promoting complex
Anaphase-promoting complex
Anaphase-Promoting Complex, also called cyclosome , is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin and RING subunit much like SCF...
via regulation of the activity of mitotic checkpoint complex. The mechanism of inhibition of APC by the mitotic checkpoint complex is poorly understood, although it is hypothesized that the MCC binds to APC as a pseudosubstrate using the KEN-box motif in BUBR1. At the same time that mitotic checkpoint complex is being activated, the centromere
Centromere
A centromere is a region of DNA typically found near the middle of a chromosome where two identical sister chromatids come closest in contact. It is involved in cell division as the point of mitotic spindle attachment...
protein CENP-E activates BUBR1, which also blocks anaphase.
Mitotic Checkpoint Complex formation
The mitotic checkpoint complex is composed of BUB3BUB3
Mitotic checkpoint protein BUB3 is a protein that in humans is encoded by the BUB3 gene.Bub3 is a protein involved with the regulation of the Spindle Assembly Checkpoint ; though BUB3 is non-essential in yeast, it is essential in higher eukaryotes...
together with MAD2 and MAD3 bound to Cdc20
CDC20
The cell-division cycle protein 20 is an essential regulator of cell division that is encoded by the CDC20 gene in humans. To the best of current knowledge its most important function is to activate the anaphase promoting complex , a large 11-13 subunit complex that initiates chromatid separation...
. MAD2 and MAD3 have distinct binding sites on CDC20, and act synergistically to inhibit APC/C. The MAD3 complex is composed of BUB3, which binds to Mad3 and BUB1B
BUB1B
Mitotic checkpoint serine/threonine-protein kinase BUB1 beta is an enzyme that in humans is encoded by the BUB1B gene.-Interactions:BUB1B has been shown to interact with AP2B1, HDAC1, BUB3, MAD2L1, Gamma-synuclein, BRCA2 and CDC20.-Further reading:...
through the short linear motif
Short linear motif
In molecular biology Short Linear Motifs are short stretches of protein sequence that mediate protein protein interaction.The first definition was given by Tim Hunt:...
known as the GLEBS motif. The exact order of attachments which must take place in order to form the MCC remains unknown. It is possible that Mad2-Cdc20 form a complex at the same time as BUBR1-BUB3-Cdc20 form another complex, and these two subcomplexes are consequently combined to form the mitotic checkpoint complex. In human cells, binding of BUBR1 to CDC20 requires prior binding of MAD2 to CDC20, so it is possible that the MAD2-CDC20 subcomplex acts as an initiator for MCC formation. BUBR1 depletion leads only to a mild reduction in Mad2-Cdc20 levels while Mad2 is required for the binding of BubR1-Bub3 to Cdc20. Nevertheless BUBR1 is still required for checkpoint activation.
The mechanism of formation for the MCC is unclear and there are competing theories for both kinetochore-dependent and kinetochore-independent formation. In support of the kinetochore-independent theory, MCC is detectable in S. cerevisiae cells in which core kinetocore assembly proteins have been mutated and cells in which the SAC has been deactivated, which suggests that the MCC could be assembled during mitosis without kinetochore localization. In one model, unattached prometaphase kinetochores can 'sensitize' APC to inhibition of MCC by recruiting the APC to kinetochores via a functioning SAC. Furthermore, depletions of various SAC proteins have revealed that MAD2 and BUBR1 depletions affect the timing of mitosis independently of kinetochores, while depletions of other SAC proteins result in a dysfunctional SAC without altering the duration of mitosis. Thus it is possible that the SAC functions through a two-stage timer where MAD2 and BUBR1 control the duration of mitosis in the first stage, which may be extended in the second stage if there are unattached kinetochores as well as other SAC proteins. However, there are lines of evidence which are in disfavor of the kinetochore-independent assembly. MCC has yet to be found during interphase
Interphase
Interphase is the phase of the cell cycle in which the cell spends the majority of its time and performs the majority of its purposes including preparation for cell division. In preparation for cell division, it increases its size and makes a copy of its DNA...
, while MCC does not form from its constituents in X. laevis meiosis II extracts without the addition of sperm of nuclei and nocodazole
Nocodazole
Nocodazole is an anti-neoplastic agent which exerts its effect in cells by interfering with the polymerization of microtubules. Microtubules are one type of fibre which constitutes the cytoskeleton, and the dynamic microtubule network has several important roles in the cell, including vesicular...
to prevent spindle assembly.
The leading model of MCC formation is the "MAD2-template model", which depends on the kinetochore dynamics of MAD2 to create the MCC. MAD1 localizes to unattached kinetochores while binding strongly to MAD2. The localization of MAD2 and BubR1 to the kinetochore may also be dependent on the Aurora B kinase
Aurora B kinase
Aurora B kinase is a protein that functions in the attachment of the mitotic spindle to the centromere.In cancerous cells, over-expression of these enzymes causes unequal distribution of genetic information, creating aneuploid cells, a hallmark of cancer....
. Cells lacking Aurora B fail to arrest in metaphase even when chromosomes lack microtubule attachment. Unattached kinetochores first bind to a MAD1-C-MAD2-p31comet complex and releases the p31comet through unknown mechanisms. The resulting MAD-C-MAD2 complex recruits the open conformer of Mad2 (O-Mad2) to the kinetochores. This O-Mad2 changes its conformation to closed Mad2 (C-Mad2) and binds Mad1. This Mad1/C-Mad2 complex is responsible for the recruitment of more O-Mad2 to the kinetochores, which changes its conformation to C-Mad2 and binds Cdc20 in an auto-amplification reaction. Since MAD1 and CDC20 both contain a similar MAD2-binding motif, the empty O-MAD2 conformation changes to C-MAD2 while binding to CDC20. This positive feedback loop is negatively regulated by p31comet, which competitively binds to C-MAD2 bound to either MAD1 or CDC20 and reduces further O-MAD2 binding to C-MAD2. Further control mechanisms may also exist, considering that p31comet is not present in lower eukaryotes. The 'template model' nomenclature is thus derived from the process where MAD1-C-MAD2 acts as a template for the formation of C-MAD2-CDC20 copies. This sequestration of Cdc20 is essential for maintaining the spindle checkpoint.
Checkpoint deactivation
Several mechanisms exist to deactivate the SAC after correct bi-orientation of sister chromatidsSister chromatids
Sister chromatids are two identical copies of a chromatid connected by a centromere. Compare sister chromatids to homologous chromosomes, which are the two different copies of the same chromosome that diploid organisms inherit, one from each parent...
. Upon microtubule-kinetochore attachment, a mechanism of stripping via a dynein-dynein motor complex
Dynein
Dynein is a motor protein in cells which converts the chemical energy contained in ATP into the mechanical energy of movement. Dynein transports various cellular cargo by "walking" along cytoskeletal microtubules towards the minus-end of the microtubule, which is usually oriented towards the cell...
transports spindle checkpoint proteins away from the kinetochores. The stripped proteins, which include MAD1, MAD2, MPS1, and CENP-F
CENPF
Centromere protein F is a protein that in humans is encoded by the CENPF gene.-Further reading:...
, are then redistributed to the spindle poles. The stripping process is highly dependent on undamaged microtubule structure as well as dynein motility along microtubules. As well as functioning as a regulator of the C-MAD2 positive feedback loop, p31comet also may act as a decativator of the SAC. Unattached kinetochores temporarily inactivate p31comet, but attachment reactivates the protein and inhibits MAD2 activation, possibly by inhibitory phosphorylation. Another possible mechanism of SAC inactivation results from energy-dependent dissociation of the MAD2-CDC20 complex through non-degradative ubiquitylation of CDC20. Conversely, the de-ubiquitylating enzyme protectin is required to maintain the SAC. Thus, unattached kinetochores maintain the checkpoint by continuously recreating the MAD2-CDC20 subcomplex from its components. The SAC may also be deativated by APC activation induced proteolysis
Proteolysis
Proteolysis is the directed degradation of proteins by cellular enzymes called proteases or by intramolecular digestion.-Purposes:Proteolysis is used by the cell for several purposes...
. Since the SAC is not reactivated by the loss of sister-chromatid cohesion during anaphase, the proteolysis of cyclin B and inactivation of the CDK1-cyclin-B kinase also inhibits SAC activity. Degradation of MPS1 during anaphase prevents the reactivation of SAC after removal of sister-chromatid cohesion. After checkpoint deactivation and during the normal anaphase of the cell cycle, the anaphase promoting complex is activated through decreasing MCC activity. When this happens the enzyme complex polyubiquitinates the anaphase inhibitor securin
Securin
Securin is a protein involved in control of the metaphase-anaphase transition and anaphase onset. Following bi-orientation of chromosome pairs and inactivation of the spindle checkpoint system, the underlying regulatory system, which includes securin, produces an abrupt stimulus that induces highly...
. The ubiquitination and destruction of securin at the end of metaphase releases the active protease called separase. Separase cleaves the cohesion molecules that hold the sister chromatids together to activate anaphase.
Spindle checkpoint defects and cancer
When the spindle checkpoint misfuctions, this can lead to chromosome missegregation, aneuploidyAneuploidy
Aneuploidy is an abnormal number of chromosomes, and is a type of chromosome abnormality. An extra or missing chromosome is a common cause of genetic disorders . Some cancer cells also have abnormal numbers of chromosomes. Aneuploidy occurs during cell division when the chromosomes do not separate...
and even tumorigenesis. Due to the fact that alterations in mitotic regulatory proteins can lead to aneuploidy and this is a frequent event in cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
, it was initially thought that these genes could be mutated in cancerous tissues. Subsequent studies in different laboratories have not found a higher frequency of mutations in these genes, although the spindle checkpoint is not working properly in many cases. What it has do been detected is that variations in the physiological levels of these proteins (such as Mad2 or BubR1) are associated with aneuploidy and tumorigenesis, and this has been demonstrated using animal model
Animal model
An animal model is a living, non-human animal used during the research and investigation of human disease, for the purpose of better understanding the disease without the added risk of causing harm to an actual human being during the process...
s.
However, recent studies indicate that what seems to happen is a more complicated scenario: aneuploidy would drive a high incidence of tumorigenesis only when alterations in the levels of specific mitotic checkpoint components (either reduction or overexpression) in tissues is also inducing other defects able to predispose them to tumors.
That is, defects such as an increase in DNA damage, chromosomal rearrangements, and/or a decreased incidence of cell death. For some mitotic checkpoint components, it is known that they are implicated in functions outside mitosis: nuclear import (Mad1), transcriptional repression (Bub3), and cell death, DNA damage response, aging, and megakaryopoiesis for BubR1. All this supports the conclusion that increase in tumorigenesis is associated with defects other than aneuploidy alone.
External links
- Ted Salmon's lab: dividing cells movies. http://www.bio.unc.edu/faculty/salmon/lab/
- Andrea Musacchio's lab: spindle checkpoint schemes. http://www.ieo-ifom-campus.it/research/musacchio.php
- http://www.uniprot.org/uniprot/O60566

