Castro-Stephens coupling
Encyclopedia
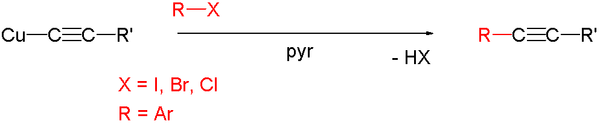
The Castro-Stephens Coupling is a cross coupling reaction between a copper(I) acetylide
and an aryl halide forming a disubstituted alkyne
and a copper(I) halide.
The reaction was discovered in 1963 by University of California, Riverside
chemists Castro and Stephens and is used as a tool in the organic synthesis
of organic compounds. The reaction has similarities with the much older Rosenmund-von Braun synthesis
(1916) between aryl halides and copper(I) cyanide
and was itself modified in 1973 with as the Sonogashira coupling
by adding a palladium catalyst and preparing the organocopper compound
in situ
, allowing copper to also be used catalytically.
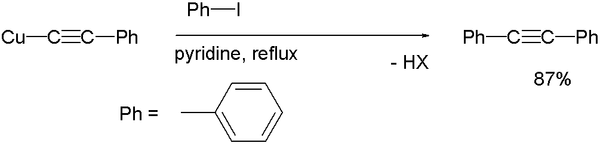
A typical reaction is the coupling of iodobenzene
with the copper acetylide of phenylacetylene
in reflux
ing pyridine
to diphenylacetylene
:
Unlike the Sonogashira coupling
, the Castro-Stephens coupling can produce heterocyclic compounds when a nucleophilic group is ortho to the aryl halide, although this typically requires use of DMF as solvent.
Copper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
and an aryl halide forming a disubstituted alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
and a copper(I) halide.
The reaction was discovered in 1963 by University of California, Riverside
University of California, Riverside
The University of California, Riverside, commonly known as UCR or UC Riverside, is a public research university and one of the ten general campuses of the University of California system. UCR is consistently ranked as one of the most ethnically and economically diverse universities in the United...
chemists Castro and Stephens and is used as a tool in the organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of organic compounds. The reaction has similarities with the much older Rosenmund-von Braun synthesis
Rosenmund-von Braun synthesis
The Rosenmund-von Braun synthesis is an organic reaction in which an aryl halide reacts with cuprous cyanide to an aryl nitrile..The reaction was named after Karl Wilhelm Rosenmund who together with his Ph.D. student Erich Struck discovered in 1914 that aryl halide reacts with alcohol water...
(1916) between aryl halides and copper(I) cyanide
Copper(I) cyanide
Copper cyanide in an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of...
and was itself modified in 1973 with as the Sonogashira coupling
Sonogashira coupling
In organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...
by adding a palladium catalyst and preparing the organocopper compound
Organocopper compound
Organocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions...
in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
, allowing copper to also be used catalytically.
A typical reaction is the coupling of iodobenzene
Iodobenzene
Iodobenzene is an organic compound consisting of a benzene ring substitituted with one iodine atom. It is useful as a synthetic intermediate in organic chemistry.-Preparation:...
with the copper acetylide of phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
in reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
ing pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
to diphenylacetylene
Diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of phenyl groups attached to both ends of an alkyne. It is a colorless crystalline material that is widely used as a building block in organic and as a ligand in organometallic chemistry.-Preparation:Several...
:
Unlike the Sonogashira coupling
Sonogashira coupling
In organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...
, the Castro-Stephens coupling can produce heterocyclic compounds when a nucleophilic group is ortho to the aryl halide, although this typically requires use of DMF as solvent.