
Resonance Raman spectroscopy
Encyclopedia
Resonance Raman spectroscopy is a specialized implementation of the more general Raman spectroscopy
.
s, and can also be used for identifying unknown substances. RR spectroscopy has found wide application to the analysis of bioinorganic molecules. Although the technique uses the same part of the electromagnetic spectrum
as infrared (IR) spectroscopy
, the two methods are actually complementary. Both are used to measure the energy
required to change the vibrational state of a chemical compound
.
IR spectroscopy involves measuring the direct absorption of photon
s with the appropriate energy to excite molecular bond
vibrations. The wavelength
s of these photons lie in the infrared
region of the spectrum
, hence the name of the technique. Raman spectroscopy measures the excitation of bond vibrations in an indirect manner. The two methods are complementary because some vibrational transitions that are observed in IR spectroscopy are not observed in Raman spectroscopy, and vice versa. RR spectroscopy
is an improvement of traditional Raman spectroscopy that has increased sensitivity and is better suited for the study of complicated systems.
X-Ray Raman Scattering
In the x-ray
region, enough energy is available for making electronic transition
s possible. At core level resonances, X-Ray Raman Scattering can become the dominating part of the x-ray fluorescence
spectrum. This is due to the resonant behavior of the Kramers-Heisenberg formula
in which the denominator is minimized for incident energies that equal a core level. This type of scattering is also known as Resonant inelastic X-ray scattering
(RIXS). In the soft x-ray range, RIXS has been shown to reflect crystal field excitations, which are often hard to observe with any other technique. Application of RIXS to strongly correlated material
s is of particular value for gaining knowledge about their electronic structure. For certain wide band materials such as graphite
, RIXS has been shown to (nearly) conserve crystal momentum and thus has found use as a complementary bandmapping technique.
. When light passes through a transparent sample, a fraction of the light is scattered in all directions. Most of the scattered photons are of the same wavelength of the incident light. This is known as Rayleigh scattering
. However, in 1928, physicists C.V. Raman and K.S. Krishnan, and independently Grigory Landsberg
and Leonid Mandelstam discovered that a small fraction of the scattered light had a different wavelength. Furthermore, this difference depended on the molecules present in the sample. For these observations, and his explanation of the phenomenon, Raman was awarded the 1930 Nobel Prize
in physics. His explanation is now known as the theory of Raman scattering
.
In the years following its discovery, Raman spectroscopy was used to provide the first catalog of molecular vibrational frequencies. Originally, heroic measures were required to obtain Raman spectra due to the low sensitivity of the technique. Typically, the sample was held in a long tube and illuminated along its length with a beam of filtered monochromatic light generated by a gas discharge lamp. The photons that were scattered by the sample were collected through an optical flat
at the end of the tube. To maximize the sensitivity, the sample was highly concentrated (1 M or more) and relatively large volumes (5 mL or more) were used. Consequently, the use of Raman spectroscopy dwindled when commercial IR spectrophotometers
became available in the 1940s. However, the advent of the laser
in the 1960s resulted in simplified Raman spectroscopy instruments and also boosted the sensitivity of the technique. This has revived the use of Raman spectroscopy as a common analytical technique.
, where N is the number of atom
s in the compound. This number arises from the ability of each atom in a molecule to move in three different directions (x, y, and z). When dealing with molecules, it is more common to consider the movement of the molecule as a whole. Consequently, the 3N degrees of freedom are partitioned into molecular translational, rotational, and vibrational motion. Three of the degrees of freedom correspond to translational motion of the molecule as a whole (along each of the three spatial dimensions). Similarly, three degrees of freedom correspond to rotations of the molecule about the ,
, 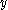 , and
, and  -axes. However, linear molecule
-axes. However, linear molecule
s only have two rotations because rotations along the bond axis do not change the positions of the atoms in the molecule. The remaining degrees of freedom correspond to molecular vibrational modes. These modes include stretching and bending motions of the chemical bond
s of the molecule. For a linear molecule, the number of vibrational modes is:
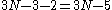
whereas for a non-linear molecule the number of vibrational modes are
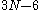
. At any given instant, each molecule in a sample has a certain amount of vibrational energy. However, the amount of vibrational energy that a molecule has continually changes due to collisions and other interactions with other molecules in the sample.
At room temperature, most of molecules will be in the lowest energy state
, which is known as the ground state
. A few molecules will be in higher energy states, which are known as excited states. The fraction of molecules occupying a given vibrational mode at a given temperature can be calculated using the Boltzmann distribution
. Performing such a calculation shows that, for relatively low temperatures (such as those used for most routine spectroscopy), most of the molecules occupy the ground vibrational state. Such a molecule can be excited to a higher vibrational mode through the direct absorption of a photon of the appropriate energy. This is the mechanism by which IR spectroscopy operates: infrared radiation is passed through the sample, and the intensity of the transmitted light is compared with that of the incident light. A reduction in intensity at a given wavelength of light indicates the absorption of energy by a vibrational transition. The energy,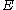 , of a photon
, of a photon
is
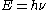
where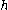 is Planck’s constant and
is Planck’s constant and  is the frequency
is the frequency
of the radiation
. Thus, the energy required for such transition may be calculated if the frequency of the incident radiation is known.
, the difference in energy between the absorbed and re-emitted photons corresponds to the energy required to excite a molecule to a higher vibrational mode.
Typically, in Raman spectroscopy high intensity laser radiation with wavelengths in either the visible or near-infrared regions of the spectrum is passed through a sample. Photons from the laser beam are absorbed by the molecules, exciting them to a virtual energy state. If the molecules relax back to the vibrational state that they started in, the reemitted photon has the same energy as the original photon. This leads to scattering of the laser light, but with no change in energy between the incoming photons and the reemitted/scattered photons. This type of scattering is known as Rayleigh scattering
.
However, it is possible for the molecules to relax back to a vibrational state that is higher in energy than the state they started in. In this case, the original photon and the reemitted photon differ in energy by the amount required to vibrationally excite the molecule. Generally, the difference in energy is recorded as the difference in wavenumber
(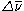 ) between the laser light and the scattered light. A Raman spectrum is generated by plotting the intensity of the reemitted light versus
) between the laser light and the scattered light. A Raman spectrum is generated by plotting the intensity of the reemitted light versus 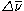 . In this example the reemitted radiation is lower in energy than the incident laser light. Consequently, the change in wavenumber is positive and results in a series of peaks in the Raman spectrum known as Stokes lines.
. In this example the reemitted radiation is lower in energy than the incident laser light. Consequently, the change in wavenumber is positive and results in a series of peaks in the Raman spectrum known as Stokes lines.
A Raman spectrum also exhibits peaks that correspond to negative values of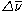 . These peaks are due to re-emitted photons that are higher in energy than the incident photons. This occurs when molecules that initially are in an excited vibrational state absorb the laser light and relax back to the lower vibrational state when they reemit the photon. These lines in the Raman spectrum are known as anti-Stokes lines. Since the Stokes lines and anti-Stokes lines gain and lose the same amount of energy, they are symmetric with respect to the peak due to elastic (Rayleigh) scattering (
. These peaks are due to re-emitted photons that are higher in energy than the incident photons. This occurs when molecules that initially are in an excited vibrational state absorb the laser light and relax back to the lower vibrational state when they reemit the photon. These lines in the Raman spectrum are known as anti-Stokes lines. Since the Stokes lines and anti-Stokes lines gain and lose the same amount of energy, they are symmetric with respect to the peak due to elastic (Rayleigh) scattering (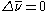 ). The anti-Stokes lines are appreciably less intense than the corresponding Stokes lines. This is because initially very few molecules are in excited vibrational states compared to the number in the ground state. Since anti-Stokes lines arise from the former and Stokes lines arise from the latter, the Stokes lines are much more intense. However, it should be noted that in molecules which exhibit fluorescence
). The anti-Stokes lines are appreciably less intense than the corresponding Stokes lines. This is because initially very few molecules are in excited vibrational states compared to the number in the ground state. Since anti-Stokes lines arise from the former and Stokes lines arise from the latter, the Stokes lines are much more intense. However, it should be noted that in molecules which exhibit fluorescence
, the Stokes lines may be obscured while the anti-Stokes lines remain unaffected. In such cases, it is necessary to use the anti-Stokes lines despite their lower intensity.
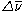 are indicative of different chemical species. This is because the frequencies of vibrational transitions depend on the atomic masses and the bond strengths. (Heavier atoms correspond to lower vibrational frequencies, while stronger bonds correspond to higher vibrational frequencies.) Thus, armed with a database of spectra from known compounds, one can unambiguously identify many different known chemical compounds based on a Raman spectrum. The number of vibrational modes scales with the number of atoms in a molecule, which means that the Raman spectra from large molecules will be very complicated. For example, protein
are indicative of different chemical species. This is because the frequencies of vibrational transitions depend on the atomic masses and the bond strengths. (Heavier atoms correspond to lower vibrational frequencies, while stronger bonds correspond to higher vibrational frequencies.) Thus, armed with a database of spectra from known compounds, one can unambiguously identify many different known chemical compounds based on a Raman spectrum. The number of vibrational modes scales with the number of atoms in a molecule, which means that the Raman spectra from large molecules will be very complicated. For example, protein
s typically contain thousands of atoms and will therefore have thousands of vibrational modes. If these modes have similar energies (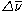 ), then the spectrum may be incredibly cluttered and complicated.
), then the spectrum may be incredibly cluttered and complicated.
Not all vibrational transitions will be “Raman active”, i.e. some vibrational transitions will not appear in the Raman spectrum. This is because of the spectroscopic selection rules for Raman. As opposed to IR spectroscopy, where a transition can only be seen when that particular vibration causes a net change in dipole moment
of the molecule, in Raman only transitions where the polarizability
of the molecule changes can be observed. This is due to the fundamental difference in how IR and Raman spectroscopy access the vibrational transitions. In Raman spectroscopy, the incoming photon causes a momentary distortion of the electron distribution around a bond in a molecule, followed by reemission of the radiation as the bond returns to its normal state. This causes temporary polarization of the bond, and an induced dipole that disappears upon relaxation. In a molecule with a center of symmetry, a change in dipole is accomplished by loss of the center of symmetry, while a change in polarizability is compatible with preservation of the center of symmetry. Thus, in a centrosymmetric molecule, asymmetrical stretching and bending will be IR active and Raman inactive, while symmetrical stretching and bending will be Raman active and IR inactive. Hence, in a centrosymmetric molecule, IR and Raman spectroscopy are mutually exclusive. For molecules without a center of symmetry, each vibrational mode may be IR active, Raman active, both, or neither. Symmetrical stretches and bends, however, tend to be Raman active.
In resonance Raman spectroscopy, the energy of the incoming laser is adjusted such that it or the scattered light coincide with an electronic transition of the molecule or crystal. In most materials the incoming and outgoing electronic resonances are sufficiently broad that they can not be distinguished. So, rather than exciting the molecule to a virtual energy state, it is excited to near one of its excited electronic transitions. Since the energy of these transitions differ from one chemical species to the next, this technique did not become applicable until the advent of tunable lasers in the early 1970s. (Tunable lasers are those where the wavelength can be altered within a specific range.) When the frequency of the laser beam is tuned to be near an electronic transition (resonance), the vibrational modes associated with that particular transition exhibit a greatly increased Raman scattering intensity. This usually overwhelms Raman signals from all of the other transitions. For instance, resonance with a π-π* transition enhances stretching modes of the π-bonds involved with the transition, while the other modes remain unaffected.
This aspect of Raman spectroscopy becomes especially useful for large biomolecules with chromophores embedded in their structure. In such chromophores, the charge-transfer (CT) transitions of the metal complex generally enhance metal-ligand
stretching modes, as well as some of modes associated with the ligands alone. Hence, in a biomolecule such as hemoglobin, tuning the laser to near the charge-transfer electronic transition of the iron center results in a spectrum reflecting only the stretching and bending modes associated with the tetrapyrrole-iron group. Consequently, in a molecule with thousands of vibrational modes, RR spectroscopy allows us to look at relatively few vibrational modes at a time. This reduces the complexity of the spectrum and allows for easier identification of an unknown protein. Also, if a protein has more than one chromophore, different chromophores can be studied individually if their CT bands differ in energy. In addition to identifying compounds, RR spectroscopy can also supply structural identification about chromophores in some cases.
The main advantage of RR spectroscopy over traditional Raman spectroscopy is the large increase in intensity of the peaks in question (by as much as a factor of 106). This allows RR spectra to be generated with sample concentrations as low as 10-8 M. This is in stark contrast to conventional Raman spectra, which usually requires concentrations greater than 0.01 M. Also, as previously mentioned, RR spectra usually exhibit only a few peaks, and different peaks can be selected for by targeting specific electronic transitions. The main disadvantage of RR spectroscopy is the increased risk of fluorescence and photodegradation of the sample due to the increased energy of the incoming laser light. Both of these factors can be minimized by using an infrared laser instead of visible light for non resonant Raman scattering, but not in RR where the laser must be tuned to the specific resonance, unless electronic levels of lower energy are available for the system under investigation.
, and other optical components. Another advantage over IR spectroscopy is that whereas water
absorbs strongly in the IR spectrum and may mask other signals, it only gives a weak signal in Raman spectroscopy. Therefore, water can easily be used as a solvent. Since lasers can be easily focused on small surface areas, the risk of sample heating and photodegradation is diminished, and the emitted radiation can be focused more efficiently. Typically, the sample is placed into a tube, which can then be spun to further decrease the sample’s exposure to the laser light, further diminishing the threat of photodegradation. Gaseous, liquid
, and solid
samples can all be analyzed using RR spectroscopy. Gas and liquid samples can be put directly into the sample chamber whereas solid samples must first be ground into a powder. With gaseous and solid samples, Raman scattering may still be too weak to easily detect. For these samples, the sample holder is placed between two mirrors that reflect the laser beam multiple times through the sample.
Since scattered light leaves the sample in all directions the probes that carry the scattered light back to the detector in Raman spectroscopy may be placed at any angle
. Usually, the detector probes are most placed at an angle of 135° to the path of the exiting laser light beam. Two other common arrangements position the detector probe at 90° or 180° with respect to the incident light. Detection angles greater than 90° are generally called back-scattering detectors because they are oriented in the same direction as the incident laser light so the radiation must scatter back to the probes. In transmitting the incident laser light to the sample and the scattered light back to the detector, fiber-optic cables may be used. Such cables can transmit light 100 m or more, thereby allowing the analysis of samples under relatively rough experimental/environmental conditions.
After the scattered radiation exits the sample, it is sent through a monochromator
. Typical monochromators consist of a diffraction grating
mounted on a rotating platform. A diffraction grating causes light dispersion
. Rotating the grating controls which wavelengths of scattered radiation reach the exit slit leading to the detector. The detector itself is usually a charge-coupled device
(CCD), which allows the entire spectrum to be recorded simultaneously. Consequently, multiple scans can be acquired in a short period of time, which can drastically increase the signal-to-noise ratio of the spectrum. Currently, Raman spectrometer
s are more expensive than more traditional dispersive instruments. As the cost of tunable lasers decrease, RR spectroscopy should see increased use, especially in the studies of metal-ligand vibrations, which reside in a region that is typically very difficult to study by other instrumental techniques. With the advent of near-infrared tunable lasers, particularly the Ti-sapphire laser (which has a range of ~700-1100 nm), Fourier Transform Resonance Raman Spectrometers may soon be commercially available. These would offer the multiplex and Jaquinot advantages of Fourier Transform
(FT) techniques.
Raman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
.
Overview
As in Raman spectroscopy, RR spectroscopy provides information about the vibrations of moleculeMolecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, and can also be used for identifying unknown substances. RR spectroscopy has found wide application to the analysis of bioinorganic molecules. Although the technique uses the same part of the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
as infrared (IR) spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
, the two methods are actually complementary. Both are used to measure the energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
required to change the vibrational state of a chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
.
IR spectroscopy involves measuring the direct absorption of photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s with the appropriate energy to excite molecular bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
vibrations. The wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s of these photons lie in the infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
region of the spectrum
Spectrum
A spectrum is a condition that is not limited to a specific set of values but can vary infinitely within a continuum. The word saw its first scientific use within the field of optics to describe the rainbow of colors in visible light when separated using a prism; it has since been applied by...
, hence the name of the technique. Raman spectroscopy measures the excitation of bond vibrations in an indirect manner. The two methods are complementary because some vibrational transitions that are observed in IR spectroscopy are not observed in Raman spectroscopy, and vice versa. RR spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
is an improvement of traditional Raman spectroscopy that has increased sensitivity and is better suited for the study of complicated systems.
X-Ray Raman Scattering
In the x-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
region, enough energy is available for making electronic transition
Molecular electronic transition
Molecular electronic transitions take place when electrons in a molecule are excited from one energy level to a higher energy level. The energy change associated with this transition provides information on the structure of a molecule and determines many molecular properties such as color...
s possible. At core level resonances, X-Ray Raman Scattering can become the dominating part of the x-ray fluorescence
X-ray fluorescence
X-ray fluorescence is the emission of characteristic "secondary" X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays...
spectrum. This is due to the resonant behavior of the Kramers-Heisenberg formula
Kramers-Heisenberg formula
The Kramers-Heisenberg dispersion formula is an expression for the cross section for scattering of a photon by an atomic electron. It was derived before the advent of quantum mechanics by Hendrik Kramers and Werner Heisenberg in 1925, based on the correspondence principle applied to the classical...
in which the denominator is minimized for incident energies that equal a core level. This type of scattering is also known as Resonant inelastic X-ray scattering
Resonant inelastic X-ray scattering
Resonant Inelastic X-ray Scattering is an x-ray spectroscopy technique used to investigate the electronic structure of molecules and materials....
(RIXS). In the soft x-ray range, RIXS has been shown to reflect crystal field excitations, which are often hard to observe with any other technique. Application of RIXS to strongly correlated material
Strongly correlated material
Strongly correlated materials are a wide class of electronic materials that show unusual electronic and magnetic properties, such as metal-insulator transitions or half-metallicity...
s is of particular value for gaining knowledge about their electronic structure. For certain wide band materials such as graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
, RIXS has been shown to (nearly) conserve crystal momentum and thus has found use as a complementary bandmapping technique.
History
Raman spectroscopy utilizes the phenomenon of scatteringScattering
Scattering is a general physical process where some forms of radiation, such as light, sound, or moving particles, are forced to deviate from a straight trajectory by one or more localized non-uniformities in the medium through which they pass. In conventional use, this also includes deviation of...
. When light passes through a transparent sample, a fraction of the light is scattered in all directions. Most of the scattered photons are of the same wavelength of the incident light. This is known as Rayleigh scattering
Rayleigh scattering
Rayleigh scattering, named after the British physicist Lord Rayleigh, is the elastic scattering of light or other electromagnetic radiation by particles much smaller than the wavelength of the light. The particles may be individual atoms or molecules. It can occur when light travels through...
. However, in 1928, physicists C.V. Raman and K.S. Krishnan, and independently Grigory Landsberg
Grigory Landsberg
Grigory Samuilovich Landsberg was a Soviet physicist.Grigory S. Landsberg is a co-discoverer of inelastic combinatorial scattering of light used now in Raman spectroscopy. His major scientific contributions were in the fields of optics and spectroscopy....
and Leonid Mandelstam discovered that a small fraction of the scattered light had a different wavelength. Furthermore, this difference depended on the molecules present in the sample. For these observations, and his explanation of the phenomenon, Raman was awarded the 1930 Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
in physics. His explanation is now known as the theory of Raman scattering
Raman scattering
Raman scattering or the Raman effect is the inelastic scattering of a photon. It was discovered by Sir Chandrasekhara Venkata Raman and Kariamanickam Srinivasa Krishnan in liquids, and by Grigory Landsberg and Leonid Mandelstam in crystals....
.
In the years following its discovery, Raman spectroscopy was used to provide the first catalog of molecular vibrational frequencies. Originally, heroic measures were required to obtain Raman spectra due to the low sensitivity of the technique. Typically, the sample was held in a long tube and illuminated along its length with a beam of filtered monochromatic light generated by a gas discharge lamp. The photons that were scattered by the sample were collected through an optical flat
Optical flat
Optical flats are optical-grade pieces of glass lapped and polished to be extremely flat on one or both sides, usually within a few millionths of an inch . They are used with a monochromatic light to determine the flatness of other optical surfaces by interference...
at the end of the tube. To maximize the sensitivity, the sample was highly concentrated (1 M or more) and relatively large volumes (5 mL or more) were used. Consequently, the use of Raman spectroscopy dwindled when commercial IR spectrophotometers
Spectrophotometry
In chemistry, spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength...
became available in the 1940s. However, the advent of the laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
in the 1960s resulted in simplified Raman spectroscopy instruments and also boosted the sensitivity of the technique. This has revived the use of Raman spectroscopy as a common analytical technique.
Degrees of Freedom
For any given chemical compound, there are a total of 3N degrees of freedomDegrees of freedom (physics and chemistry)
A degree of freedom is an independent physical parameter, often called a dimension, in the formal description of the state of a physical system...
, where N is the number of atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s in the compound. This number arises from the ability of each atom in a molecule to move in three different directions (x, y, and z). When dealing with molecules, it is more common to consider the movement of the molecule as a whole. Consequently, the 3N degrees of freedom are partitioned into molecular translational, rotational, and vibrational motion. Three of the degrees of freedom correspond to translational motion of the molecule as a whole (along each of the three spatial dimensions). Similarly, three degrees of freedom correspond to rotations of the molecule about the
 ,
, 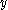 , and
, and  -axes. However, linear molecule
-axes. However, linear moleculeChromonic
In a chromonic, relatively flat molecules form linear aggregates. For aqueous solutions, the molecules have a flat core, such as a carbon ring system, with highly water-soluble side groups, such as a sulfonate or carboxyl. The molecules aggregate in linear stacks with the flat cores lying one on...
s only have two rotations because rotations along the bond axis do not change the positions of the atoms in the molecule. The remaining degrees of freedom correspond to molecular vibrational modes. These modes include stretching and bending motions of the chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s of the molecule. For a linear molecule, the number of vibrational modes is:
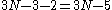
whereas for a non-linear molecule the number of vibrational modes are
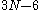
Molecular Vibrations and Infrared Radiation
The frequencies of molecular vibrations range from less than 1012 to approximately 1014 Hz. These frequencies correspond to radiation in the infrared (IR) region of the electromagnetic spectrumElectromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
. At any given instant, each molecule in a sample has a certain amount of vibrational energy. However, the amount of vibrational energy that a molecule has continually changes due to collisions and other interactions with other molecules in the sample.
At room temperature, most of molecules will be in the lowest energy state
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
, which is known as the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
. A few molecules will be in higher energy states, which are known as excited states. The fraction of molecules occupying a given vibrational mode at a given temperature can be calculated using the Boltzmann distribution
Boltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
. Performing such a calculation shows that, for relatively low temperatures (such as those used for most routine spectroscopy), most of the molecules occupy the ground vibrational state. Such a molecule can be excited to a higher vibrational mode through the direct absorption of a photon of the appropriate energy. This is the mechanism by which IR spectroscopy operates: infrared radiation is passed through the sample, and the intensity of the transmitted light is compared with that of the incident light. A reduction in intensity at a given wavelength of light indicates the absorption of energy by a vibrational transition. The energy,
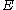 , of a photon
, of a photonPhoton
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
is
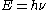
where
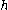 is Planck’s constant and
is Planck’s constant and  is the frequency
is the frequencyFrequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the radiation
Radiation
In physics, radiation is a process in which energetic particles or energetic waves travel through a medium or space. There are two distinct types of radiation; ionizing and non-ionizing...
. Thus, the energy required for such transition may be calculated if the frequency of the incident radiation is known.
Raman Scattering
It is also possible to observe molecular vibrations by an inelastic scattering process. In inelastic scattering, an absorbed photon is re-emitted with lower energy. In Raman scatteringRaman scattering
Raman scattering or the Raman effect is the inelastic scattering of a photon. It was discovered by Sir Chandrasekhara Venkata Raman and Kariamanickam Srinivasa Krishnan in liquids, and by Grigory Landsberg and Leonid Mandelstam in crystals....
, the difference in energy between the absorbed and re-emitted photons corresponds to the energy required to excite a molecule to a higher vibrational mode.
Typically, in Raman spectroscopy high intensity laser radiation with wavelengths in either the visible or near-infrared regions of the spectrum is passed through a sample. Photons from the laser beam are absorbed by the molecules, exciting them to a virtual energy state. If the molecules relax back to the vibrational state that they started in, the reemitted photon has the same energy as the original photon. This leads to scattering of the laser light, but with no change in energy between the incoming photons and the reemitted/scattered photons. This type of scattering is known as Rayleigh scattering
Rayleigh scattering
Rayleigh scattering, named after the British physicist Lord Rayleigh, is the elastic scattering of light or other electromagnetic radiation by particles much smaller than the wavelength of the light. The particles may be individual atoms or molecules. It can occur when light travels through...
.
However, it is possible for the molecules to relax back to a vibrational state that is higher in energy than the state they started in. In this case, the original photon and the reemitted photon differ in energy by the amount required to vibrationally excite the molecule. Generally, the difference in energy is recorded as the difference in wavenumber
Wavenumber
In the physical sciences, the wavenumber is a property of a wave, its spatial frequency, that is proportional to the reciprocal of the wavelength. It is also the magnitude of the wave vector...
(
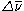 ) between the laser light and the scattered light. A Raman spectrum is generated by plotting the intensity of the reemitted light versus
) between the laser light and the scattered light. A Raman spectrum is generated by plotting the intensity of the reemitted light versus 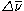 . In this example the reemitted radiation is lower in energy than the incident laser light. Consequently, the change in wavenumber is positive and results in a series of peaks in the Raman spectrum known as Stokes lines.
. In this example the reemitted radiation is lower in energy than the incident laser light. Consequently, the change in wavenumber is positive and results in a series of peaks in the Raman spectrum known as Stokes lines.A Raman spectrum also exhibits peaks that correspond to negative values of
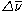 . These peaks are due to re-emitted photons that are higher in energy than the incident photons. This occurs when molecules that initially are in an excited vibrational state absorb the laser light and relax back to the lower vibrational state when they reemit the photon. These lines in the Raman spectrum are known as anti-Stokes lines. Since the Stokes lines and anti-Stokes lines gain and lose the same amount of energy, they are symmetric with respect to the peak due to elastic (Rayleigh) scattering (
. These peaks are due to re-emitted photons that are higher in energy than the incident photons. This occurs when molecules that initially are in an excited vibrational state absorb the laser light and relax back to the lower vibrational state when they reemit the photon. These lines in the Raman spectrum are known as anti-Stokes lines. Since the Stokes lines and anti-Stokes lines gain and lose the same amount of energy, they are symmetric with respect to the peak due to elastic (Rayleigh) scattering (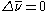 ). The anti-Stokes lines are appreciably less intense than the corresponding Stokes lines. This is because initially very few molecules are in excited vibrational states compared to the number in the ground state. Since anti-Stokes lines arise from the former and Stokes lines arise from the latter, the Stokes lines are much more intense. However, it should be noted that in molecules which exhibit fluorescence
). The anti-Stokes lines are appreciably less intense than the corresponding Stokes lines. This is because initially very few molecules are in excited vibrational states compared to the number in the ground state. Since anti-Stokes lines arise from the former and Stokes lines arise from the latter, the Stokes lines are much more intense. However, it should be noted that in molecules which exhibit fluorescenceFluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
, the Stokes lines may be obscured while the anti-Stokes lines remain unaffected. In such cases, it is necessary to use the anti-Stokes lines despite their lower intensity.
Resonance Raman Spectroscopy
Raman spectroscopy can be used to identify chemical compounds because the values of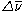 are indicative of different chemical species. This is because the frequencies of vibrational transitions depend on the atomic masses and the bond strengths. (Heavier atoms correspond to lower vibrational frequencies, while stronger bonds correspond to higher vibrational frequencies.) Thus, armed with a database of spectra from known compounds, one can unambiguously identify many different known chemical compounds based on a Raman spectrum. The number of vibrational modes scales with the number of atoms in a molecule, which means that the Raman spectra from large molecules will be very complicated. For example, protein
are indicative of different chemical species. This is because the frequencies of vibrational transitions depend on the atomic masses and the bond strengths. (Heavier atoms correspond to lower vibrational frequencies, while stronger bonds correspond to higher vibrational frequencies.) Thus, armed with a database of spectra from known compounds, one can unambiguously identify many different known chemical compounds based on a Raman spectrum. The number of vibrational modes scales with the number of atoms in a molecule, which means that the Raman spectra from large molecules will be very complicated. For example, proteinProtein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s typically contain thousands of atoms and will therefore have thousands of vibrational modes. If these modes have similar energies (
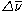 ), then the spectrum may be incredibly cluttered and complicated.
), then the spectrum may be incredibly cluttered and complicated.Not all vibrational transitions will be “Raman active”, i.e. some vibrational transitions will not appear in the Raman spectrum. This is because of the spectroscopic selection rules for Raman. As opposed to IR spectroscopy, where a transition can only be seen when that particular vibration causes a net change in dipole moment
Bond dipole moment
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
of the molecule, in Raman only transitions where the polarizability
Polarizability
Polarizability is the measure of the change in a molecule's electron distribution in response to an applied electric field, which can also be induced by electric interactions with solvents or ionic reagents. It is a property of matter...
of the molecule changes can be observed. This is due to the fundamental difference in how IR and Raman spectroscopy access the vibrational transitions. In Raman spectroscopy, the incoming photon causes a momentary distortion of the electron distribution around a bond in a molecule, followed by reemission of the radiation as the bond returns to its normal state. This causes temporary polarization of the bond, and an induced dipole that disappears upon relaxation. In a molecule with a center of symmetry, a change in dipole is accomplished by loss of the center of symmetry, while a change in polarizability is compatible with preservation of the center of symmetry. Thus, in a centrosymmetric molecule, asymmetrical stretching and bending will be IR active and Raman inactive, while symmetrical stretching and bending will be Raman active and IR inactive. Hence, in a centrosymmetric molecule, IR and Raman spectroscopy are mutually exclusive. For molecules without a center of symmetry, each vibrational mode may be IR active, Raman active, both, or neither. Symmetrical stretches and bends, however, tend to be Raman active.
In resonance Raman spectroscopy, the energy of the incoming laser is adjusted such that it or the scattered light coincide with an electronic transition of the molecule or crystal. In most materials the incoming and outgoing electronic resonances are sufficiently broad that they can not be distinguished. So, rather than exciting the molecule to a virtual energy state, it is excited to near one of its excited electronic transitions. Since the energy of these transitions differ from one chemical species to the next, this technique did not become applicable until the advent of tunable lasers in the early 1970s. (Tunable lasers are those where the wavelength can be altered within a specific range.) When the frequency of the laser beam is tuned to be near an electronic transition (resonance), the vibrational modes associated with that particular transition exhibit a greatly increased Raman scattering intensity. This usually overwhelms Raman signals from all of the other transitions. For instance, resonance with a π-π* transition enhances stretching modes of the π-bonds involved with the transition, while the other modes remain unaffected.
This aspect of Raman spectroscopy becomes especially useful for large biomolecules with chromophores embedded in their structure. In such chromophores, the charge-transfer (CT) transitions of the metal complex generally enhance metal-ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
stretching modes, as well as some of modes associated with the ligands alone. Hence, in a biomolecule such as hemoglobin, tuning the laser to near the charge-transfer electronic transition of the iron center results in a spectrum reflecting only the stretching and bending modes associated with the tetrapyrrole-iron group. Consequently, in a molecule with thousands of vibrational modes, RR spectroscopy allows us to look at relatively few vibrational modes at a time. This reduces the complexity of the spectrum and allows for easier identification of an unknown protein. Also, if a protein has more than one chromophore, different chromophores can be studied individually if their CT bands differ in energy. In addition to identifying compounds, RR spectroscopy can also supply structural identification about chromophores in some cases.
The main advantage of RR spectroscopy over traditional Raman spectroscopy is the large increase in intensity of the peaks in question (by as much as a factor of 106). This allows RR spectra to be generated with sample concentrations as low as 10-8 M. This is in stark contrast to conventional Raman spectra, which usually requires concentrations greater than 0.01 M. Also, as previously mentioned, RR spectra usually exhibit only a few peaks, and different peaks can be selected for by targeting specific electronic transitions. The main disadvantage of RR spectroscopy is the increased risk of fluorescence and photodegradation of the sample due to the increased energy of the incoming laser light. Both of these factors can be minimized by using an infrared laser instead of visible light for non resonant Raman scattering, but not in RR where the laser must be tuned to the specific resonance, unless electronic levels of lower energy are available for the system under investigation.
Instrumentation
In RR spectroscopy, the light source consists of a tunable laser, whose radiation lies in either the near-infrared, visible, or near-ultraviolet regions of the spectrum. In creating a sample handling system, RR spectroscopy offers an advantage over IR spectroscopy in that glass can be used for windows, lensesLens (optics)
A lens is an optical device with perfect or approximate axial symmetry which transmits and refracts light, converging or diverging the beam. A simple lens consists of a single optical element...
, and other optical components. Another advantage over IR spectroscopy is that whereas water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
absorbs strongly in the IR spectrum and may mask other signals, it only gives a weak signal in Raman spectroscopy. Therefore, water can easily be used as a solvent. Since lasers can be easily focused on small surface areas, the risk of sample heating and photodegradation is diminished, and the emitted radiation can be focused more efficiently. Typically, the sample is placed into a tube, which can then be spun to further decrease the sample’s exposure to the laser light, further diminishing the threat of photodegradation. Gaseous, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
, and solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
samples can all be analyzed using RR spectroscopy. Gas and liquid samples can be put directly into the sample chamber whereas solid samples must first be ground into a powder. With gaseous and solid samples, Raman scattering may still be too weak to easily detect. For these samples, the sample holder is placed between two mirrors that reflect the laser beam multiple times through the sample.
Since scattered light leaves the sample in all directions the probes that carry the scattered light back to the detector in Raman spectroscopy may be placed at any angle
Angle
In geometry, an angle is the figure formed by two rays sharing a common endpoint, called the vertex of the angle.Angles are usually presumed to be in a Euclidean plane with the circle taken for standard with regard to direction. In fact, an angle is frequently viewed as a measure of an circular arc...
. Usually, the detector probes are most placed at an angle of 135° to the path of the exiting laser light beam. Two other common arrangements position the detector probe at 90° or 180° with respect to the incident light. Detection angles greater than 90° are generally called back-scattering detectors because they are oriented in the same direction as the incident laser light so the radiation must scatter back to the probes. In transmitting the incident laser light to the sample and the scattered light back to the detector, fiber-optic cables may be used. Such cables can transmit light 100 m or more, thereby allowing the analysis of samples under relatively rough experimental/environmental conditions.
After the scattered radiation exits the sample, it is sent through a monochromator
Monochromator
A monochromator is an optical device that transmits a mechanically selectable narrow band of wavelengths of light or other radiation chosen from a wider range of wavelengths available at the input...
. Typical monochromators consist of a diffraction grating
Diffraction grating
In optics, a diffraction grating is an optical component with a periodic structure, which splits and diffracts light into several beams travelling in different directions. The directions of these beams depend on the spacing of the grating and the wavelength of the light so that the grating acts as...
mounted on a rotating platform. A diffraction grating causes light dispersion
Dispersion
Dispersion may refer to:In physics:*The dependence of wave velocity on frequency or wavelength:**Dispersion , for light waves**Dispersion **Acoustic dispersion, for sound waves...
. Rotating the grating controls which wavelengths of scattered radiation reach the exit slit leading to the detector. The detector itself is usually a charge-coupled device
Charge-coupled device
A charge-coupled device is a device for the movement of electrical charge, usually from within the device to an area where the charge can be manipulated, for example conversion into a digital value. This is achieved by "shifting" the signals between stages within the device one at a time...
(CCD), which allows the entire spectrum to be recorded simultaneously. Consequently, multiple scans can be acquired in a short period of time, which can drastically increase the signal-to-noise ratio of the spectrum. Currently, Raman spectrometer
Spectrometer
A spectrometer is an instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify materials. The variable measured is most often the light's intensity but could also, for instance, be the polarization...
s are more expensive than more traditional dispersive instruments. As the cost of tunable lasers decrease, RR spectroscopy should see increased use, especially in the studies of metal-ligand vibrations, which reside in a region that is typically very difficult to study by other instrumental techniques. With the advent of near-infrared tunable lasers, particularly the Ti-sapphire laser (which has a range of ~700-1100 nm), Fourier Transform Resonance Raman Spectrometers may soon be commercially available. These would offer the multiplex and Jaquinot advantages of Fourier Transform
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
(FT) techniques.
External links
- Resonance Raman Theory Resource connects Kramers-Heisenberg dispersion formula to time-dependent perturbation theory

