Pictet-Spengler reaction
Encyclopedia
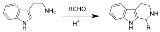
The Pictet–Spengler reaction is a chemical reaction
in which a β-arylethylamine such as tryptamine
undergoes ringclosure after condensation
with an aldehyde
or ketone
. Usually an acidic catalyst is employed and the reaction mixture heated, but some reactive compounds give good yields even at physiologic conditions. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction
.
 The reaction was discovered in 1911 by Amé Pictet
The reaction was discovered in 1911 by Amé Pictet
and Theodor Spengler. It has remained an important reaction in the fields of alkaloid
and pharmaceutical synthesis
. The Pictet–Spengler reaction product of tryptophan
and aldose
s can be identified in foodstuffs such as soy sauce
and ketchup
.
Nucleophilic aromatic rings such as indole
or pyrrole
give products with good yields and mild conditions, while less nucleophilic aromatic rings such as phenyl give poor yields despite high temperatures and strong acid. The original Pictet–Spengler reaction was the reaction of β-phenethylamine
with the dimethyl acetal
of formaldehyde
and hydrochloric acid
forming a tetrahydroisoquinoline
.
Like the Mannich reaction, aldehydes give good yields while ketones tend to give lower conversion.
The Pictet–Spengler reaction has been applied to solid-phase
combinatorial chemistry
with great success.
An analogous reaction with an aryl-β-ethanol is called Oxa-Pictet–Spengler reaction
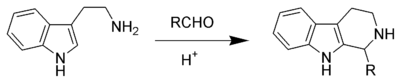
double bond that is the driving force of the cyclization. The reaction mechanism
occurs by initial formation of an iminium ion (4) followed by electrophilic substitution
at the 2-position. After deprotonation, the desired product (6) is formed. The reaction shown is an example of a 6-endo-trig reaction, which is favoured by Baldwin's ring closure rules
.
, trifluoroacetic acid
or superacid
s.
the iminium ion forming the intermediate N-acyliminium ion. The N-acyliminium ion is a very powerful electrophile
and most aromatic ring systems will cyclize under mild conditions with good yields.
 Tadalafil
Tadalafil
is synthesized via the N-acyliminium Pictet–Spengler reaction. This reaction can also be catalyzed by AuCl3 and AgOTf.
, a new chiral center is created. Several substrate- or auxiliary-controlled diastereoselective Pictet–Spengler reactions have been developed. Additionally, Seayad et al. have published a chiral Lewis acid
that catalyzes asymmetric Pictet–Spengler reactions.
Tryptophans: Diastereocontrolled reaction
The reaction of enantiopure tryptophan
or its short-chain alkylester
s leads to 1,2,3,4-tetrahydro-β-carbolines in which a new chiral
center at C-1 adopts either a cis
or trans configuration
towards the C-3 carboxyl group. The cis conduction is kinetic
ally controlled, i.e. it is performed at lower temperatures. At higher temperatures the reaction becomes reversible and usually favours racemisation. 1,3-trans dominated products can be obtained with Nb-benzyl
ated tryptophans, which are accessible by reductive amination
. The benzyl group can be removed hydrogenolytically
afterwards. As a rough rule, 13C NMR
signals for C1 and C3 are downfield shifted in cis products relative to trans products (see steric compression effect).
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in which a β-arylethylamine such as tryptamine
Tryptamine
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
undergoes ringclosure after condensation
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
with an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. Usually an acidic catalyst is employed and the reaction mixture heated, but some reactive compounds give good yields even at physiologic conditions. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
.

Amé Pictet
Amé Pictet was a Swiss chemist. He discovered the Pictet-Spengler reaction.Pictet was born in Geneva, studied with August Kekulé at the University of Bonn where he received his Ph.D in 1879. From 1894 til 1932 he was professor at the University of Geneva. Pictet died in Geneva in 1937.-External...
and Theodor Spengler. It has remained an important reaction in the fields of alkaloid
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
and pharmaceutical synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. The Pictet–Spengler reaction product of tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
and aldose
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
s can be identified in foodstuffs such as soy sauce
Soy sauce
Soy sauce is a condiment produced by fermenting soybeans with Aspergillus oryzae or Aspergillus sojae molds, along with water and salt...
and ketchup
Ketchup
Ketchup is a sweet-and-tangy condiment typically made from tomatoes, vinegar, sugar or high-fructose corn syrup and an assortment of...
.
Nucleophilic aromatic rings such as indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
or pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
give products with good yields and mild conditions, while less nucleophilic aromatic rings such as phenyl give poor yields despite high temperatures and strong acid. The original Pictet–Spengler reaction was the reaction of β-phenethylamine
Phenethylamine
Phenylethylamine or phenethylamine is a natural monoamine alkaloid, trace amine, and also the name of a class of chemicals with many members well known for psychoactive drug and stimulant effects. Studies suggest that phenylethylamine functions as a neuromodulator or neurotransmitter in the...
with the dimethyl acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
of formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
and hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
forming a tetrahydroisoquinoline
Tetrahydroisoquinoline
Tetrahydroisoquinoline is a secondary amine with the chemical formula C9H11N.-Reactions:Like other secondary amines, tetrahydroisoquinoline can be oxidized to the corresponding nitrone using hydrogen peroxide, catalyzed by selenium dioxide....
.
Like the Mannich reaction, aldehydes give good yields while ketones tend to give lower conversion.
The Pictet–Spengler reaction has been applied to solid-phase
Solid-phase synthesis
In chemistry, solid-phase synthesis is a method in which molecules are bound on a bead and synthesized step-by-step in a reactant solution; compared with normal synthesis in a liquid state, it is easier to remove excess reactant or byproduct from the product. In this method, building blocks are...
combinatorial chemistry
Combinatorial chemistry
Combinatorial chemistry involves the rapid synthesis or the computer simulation of a large number of different but structurally related molecules or materials...
with great success.
An analogous reaction with an aryl-β-ethanol is called Oxa-Pictet–Spengler reaction
Reaction mechanism
It is the electrophilicity of the imineImine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
double bond that is the driving force of the cyclization. The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
occurs by initial formation of an iminium ion (4) followed by electrophilic substitution
Electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a group in a compound, typically but not always hydrogen. Electrophilic aromatic substitution is characteristic of aromatic compounds and is an important way of introducing functional groups onto benzene...
at the 2-position. After deprotonation, the desired product (6) is formed. The reaction shown is an example of a 6-endo-trig reaction, which is favoured by Baldwin's ring closure rules
Baldwin's rules
Baldwin's Rules in organic chemistry are a series of guidelines outlining the relative favourabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976...
.

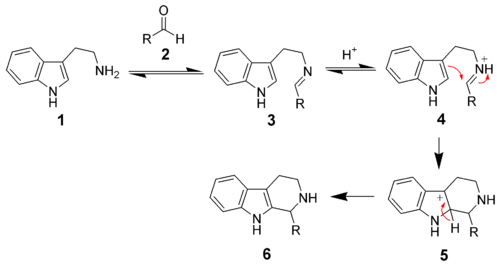
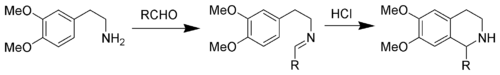
Pictet-Spengler tetrahydroisoquinoline synthesis
Replacing an indole with a 3,4-dimethoxyphenyl group give the reaction named the Pictet-Spengler tetrahydroisoquinoline synthesis. Reaction conditions are generally harsher than the indole variant, and require refluxing conditions with strong acids like hydrochloric acidHydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
or superacid
Superacid
According to the classical definition superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function of −12. According to the modern definition, superacid is a medium, in which the chemical potential of the proton is higher than in pure...
s.

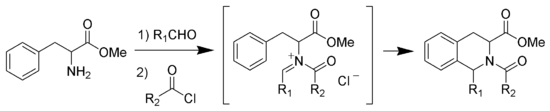
N-Acyliminium Pictet-Spengler Reaction
Instead of catalyzing the Pictet-Spengler cyclization with strong acid, one can acylateAcylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
the iminium ion forming the intermediate N-acyliminium ion. The N-acyliminium ion is a very powerful electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
and most aromatic ring systems will cyclize under mild conditions with good yields.

Tadalafil
Tadalafil is a PDE5 inhibitor, currently marketed in pill form for treating erectile dysfunction under the name Cialis; and under the name Adcirca for the treatment of pulmonary arterial hypertension...
is synthesized via the N-acyliminium Pictet–Spengler reaction. This reaction can also be catalyzed by AuCl3 and AgOTf.
Asymmetric Pictet-Spengler reaction
When the Pictet–Spengler reaction is done with an aldehyde other than formaldehydeFormaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
, a new chiral center is created. Several substrate- or auxiliary-controlled diastereoselective Pictet–Spengler reactions have been developed. Additionally, Seayad et al. have published a chiral Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
that catalyzes asymmetric Pictet–Spengler reactions.
Tryptophans: Diastereocontrolled reaction
The reaction of enantiopure tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
or its short-chain alkylester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s leads to 1,2,3,4-tetrahydro-β-carbolines in which a new chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
center at C-1 adopts either a cis
Geometric isomerism
In organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
or trans configuration
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
towards the C-3 carboxyl group. The cis conduction is kinetic
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
ally controlled, i.e. it is performed at lower temperatures. At higher temperatures the reaction becomes reversible and usually favours racemisation. 1,3-trans dominated products can be obtained with Nb-benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
ated tryptophans, which are accessible by reductive amination
Reductive amination
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine...
. The benzyl group can be removed hydrogenolytically
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
afterwards. As a rough rule, 13C NMR
Carbon-13 NMR
Carbon-13 NMR is the application of nuclear magnetic resonance spectroscopy to carbon. It is analogous to proton NMR and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms...
signals for C1 and C3 are downfield shifted in cis products relative to trans products (see steric compression effect).

