Horner-Wadsworth-Emmons reaction
Encyclopedia
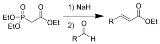
The Horner-Wadsworth-Emmons reaction (or HWE reaction) is the chemical reaction
of stabilized phosphonate
carbanion
s with aldehyde
s (or ketone
s) to produce predominantly E-alkene
s.
 In 1958, Leopold Horner
In 1958, Leopold Horner
published a modified Wittig reaction
using phosphonate-stabilized carbanions. William S. Wadsworth and William D. Emmons
further defined the reaction.
In contrast to phosphonium ylides used in the Wittig reaction
, phosphonate-stabilized carbanions are more nucleophilic and more basic. Likewise, phosphonate-stabilized carbanions can be alkylated, unlike phosphonium ylides the dialkylphosphate salt byproduct is easily removed by aqueous extraction
.
Several reviews have been published.
of the phosphonate
to give the phosphonate carbanion
1. Nucleophilic addition
of the carbanion onto the aldehyde 2 (or ketone) producing 3a or 3b is the rate-limiting step. If R2=H, then intermediates 3a and 4a and intermediates 3b and 4b can interconvert with each other. The final elimination
of 4a and 4b yield E-alkene 5 and Z-alkene 6.
 The ratio of alkene isomer
The ratio of alkene isomer
s 5 and 6 is dependent upon the stereochemical outcome of the initial carbanion addition and upon the ability of the intermediates to equilibrate
.
The electron-withdrawing group (EWG) alpha to the phosphonate is necessary for the final elimination to occur. In the absence of an electron-withdrawing group, the final product is the α-hydroxyphosphonate 3a and 3b. However, these α-hydroxyphosphonates can be transformed to alkene
s by reaction with diisopropylcarbodiimide.
have performed a systematic study of the reaction of trimethyl phosphonoacetate with various aldehydes. While each effect was small, they had a cumulative effect making it possible to modify the stereochemical outcome without modifying the structure of the phosphonate. They found greater E-stereoselectivity with the following conditions:
In a separate study, it was found that bulky phosphonate and bulky electron-withdrawing groups enhance E-alkene selectivity.

Aromatic aldehydes produce almost exclusively E-alkenes. In case Z-alkenes from aromatic aldehydes are needed, the Still modification (see below) can be used.
s is poor to modest.
, several procedures have been developed using milder bases. Masamune and Roush have developed mild conditions using lithium chloride
and DBU
. Rathke extended this to lithium
or magnesium
halide
s with triethylamine
. Several other bases have been found effective.
in THF
) nearly exclusive Z-alkene production can be achieved.
 Ando has suggested that the use of electron-deficient phosphonates accelerates the elimination of the oxaphosphatane intermediates.
Ando has suggested that the use of electron-deficient phosphonates accelerates the elimination of the oxaphosphatane intermediates.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of stabilized phosphonate
Phosphonate
Phosphonates or phosphonic acids are organic compounds containing C-PO2 or C-PO2 groups . Bisphosphonates were first synthesized in 1897 by Von Baeyer and Hofmann. An example of such a bisphosphonate is HEDP . Since the work of Schwarzenbach in 1949, phosphonic acids are known as effective...
carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
s with aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s (or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s) to produce predominantly E-alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s.

Leopold Horner
Leopold Horner was a German chemist and published in a modified Wittig reaction using phosphonate-stabilized carbanions now called the Horner-Wadsworth-Emmons reaction or HWE reaction or Horner-Wittig reaction-Life:Horner started studying chemistry at the University of Heidelberg and later with...
published a modified Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
using phosphonate-stabilized carbanions. William S. Wadsworth and William D. Emmons
William D. Emmons
William D. Emmons was an American chemist and published together William S. Wadsworth a modifications to the Wittig-Horner reaction using phosphonate-stabilized carbanions now called the Horner-Wadsworth-Emmons reaction or HWE reaction or Horner-Wittig reaction-Life:Emmons studied at the...
further defined the reaction.
In contrast to phosphonium ylides used in the Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
, phosphonate-stabilized carbanions are more nucleophilic and more basic. Likewise, phosphonate-stabilized carbanions can be alkylated, unlike phosphonium ylides the dialkylphosphate salt byproduct is easily removed by aqueous extraction
Liquid-liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid...
.
Several reviews have been published.
Reaction mechanism
The Horner-Wadsworth-Emmons reaction begins with the deprotonationDeprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of the phosphonate
Phosphonate
Phosphonates or phosphonic acids are organic compounds containing C-PO2 or C-PO2 groups . Bisphosphonates were first synthesized in 1897 by Von Baeyer and Hofmann. An example of such a bisphosphonate is HEDP . Since the work of Schwarzenbach in 1949, phosphonic acids are known as effective...
to give the phosphonate carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
1. Nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of the carbanion onto the aldehyde 2 (or ketone) producing 3a or 3b is the rate-limiting step. If R2=H, then intermediates 3a and 4a and intermediates 3b and 4b can interconvert with each other. The final elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of 4a and 4b yield E-alkene 5 and Z-alkene 6.

Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s 5 and 6 is dependent upon the stereochemical outcome of the initial carbanion addition and upon the ability of the intermediates to equilibrate
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
.
The electron-withdrawing group (EWG) alpha to the phosphonate is necessary for the final elimination to occur. In the absence of an electron-withdrawing group, the final product is the α-hydroxyphosphonate 3a and 3b. However, these α-hydroxyphosphonates can be transformed to alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s by reaction with diisopropylcarbodiimide.
Stereoselectivity
The Horner-Wadsworth-Emmons reaction favours the formation of E-alkenes. In general, the more equilibration amongst intermediates, the higher the selectivity for E-alkene formation.Disubstituted alkenes
Thompson and HeathcockClayton Heathcock
Clayton Heathcock is an organic chemist, Professor of Chemistry, and Dean of the College of Chemistry at the University of California, Berkeley. Professor Heathcock is well known for his accomplishments in the synthesis of complex polycyclic natural products and for his contributions to the...
have performed a systematic study of the reaction of trimethyl phosphonoacetate with various aldehydes. While each effect was small, they had a cumulative effect making it possible to modify the stereochemical outcome without modifying the structure of the phosphonate. They found greater E-stereoselectivity with the following conditions:
- Increasing steric bulk of the aldehyde
- Higher reaction temperatures (23 °C over -78 °C)
- LiLithiumLithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
> NaSodiumSodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
> KPotassiumPotassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
salts - Using the solvent DMEDimethoxyethaneDimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a clear, colorless, aprotic, and liquid ether that is used as a solvent. Dimethoxyethane is miscible with water.Dimethoxyethane is often used as a higher boiling...
over THFTetrahydrofuranTetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
In a separate study, it was found that bulky phosphonate and bulky electron-withdrawing groups enhance E-alkene selectivity.
Trisubstituted alkenes
The steric bulk of the phosphonate and electron-withdrawing groups plays a critical role in the reaction of α-branched phosphonates with aliphatic aldehydes.
| R1 | R2 | Ratio of alkenes ( E : Z ) |
|---|---|---|
| Methyl | Methyl | 5 : 95 |
| Methyl | Ethyl | 10 : 90 |
| Ethyl Ethyl group In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5.... |
Ethyl | 40 : 60 |
| Isopropyl Isopropyl In organic chemistry, isopropyl is a propyl with a group attached to the secondary carbon. If viewed as a functional group an isopropyl is an organic compound with a propyl group attached at its secondary carbon.The bond is therefore on the middle carbon.... |
Ethyl | 90 : 10 |
| Isopropyl | Isopropyl | 95 : 5 |
Aromatic aldehydes produce almost exclusively E-alkenes. In case Z-alkenes from aromatic aldehydes are needed, the Still modification (see below) can be used.
Olefination of ketones
The stereoselectivity of the Horner-Wadsworth-Emmons reaction of ketoneKetone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s is poor to modest.
Base sensitive substrates
Since many substrates are not stable to sodium hydrideSodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
, several procedures have been developed using milder bases. Masamune and Roush have developed mild conditions using lithium chloride
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
and DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
. Rathke extended this to lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
or magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s with triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
. Several other bases have been found effective.
Still modification
Still and Gennari have developed conditions that give Z-alkenes with excellent stereoselectivity. Using phosphonates with electron-withdrawing groups (trifluoroethyl) together with strongly dissociating conditions (KHMDS and 18-crown-618-Crown-6
18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. The compound is a crown ether. Crown ethers coordinate some metal cations in their central cavity; 18-crown-6 displays a particular affinity for potassium cations. The synthesis...
in THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
) nearly exclusive Z-alkene production can be achieved.

See also
- Michaelis–Arbuzov reaction
- Michaelis–Becker reactionMichaelis–Becker reactionThe Michaelis–Becker reaction is the reaction of a hydrogen phosphonate with a base, followed by a nucleophilic substitution of phosphorus on an alkyl halide, to afford an alkyl phosphonate. Yields of this reaction are often lower than the corresponding Michaelis-Arbuzov reaction....
- Peterson reaction
- Tebbe olefination

