Metastability
Encyclopedia
Complex systems
Complex systems present problems in mathematical modelling.The equations from which complex system models are developed generally derive from statistical physics, information theory and non-linear dynamics, and represent organized but unpredictable behaviors of systems of nature that are considered...
when leaving their most stable state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
after an external action.
The parameters of such "excited" systems may eventually reach and hold stationary values (a metastable state) but then, after a long time, (spontaneously or under a slight external action) parameters will start changing again.
In terms of classical mechanics, a ball rolling down a slope and then fully stopping on the edge of a ledge may start rolling again to lower levels as result of small catastrophic rearrangements
Catastrophe theory
In mathematics, catastrophe theory is a branch of bifurcation theory in the study of dynamical systems; it is also a particular special case of more general singularity theory in geometry....
.
The metastability concept originates in the physics of first-order phase transitions later to acquire new meanings in the study of aggregated subatomic particle
Subatomic particle
In physics or chemistry, subatomic particles are the smaller particles composing nucleons and atoms. There are two types of subatomic particles: elementary particles, which are not made of other particles, and composite particles...
s (in atomic nuclei or in atoms) or in molecules, macromolecules or clusters of atoms and molecules. Later on it was borrowed for the study of decision-making and information transmitting systems.
Many complex natural and man-made systems can demonstrate metastability.
- In physicsPhysicsPhysics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
and chemistryChemistryChemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
it is most prevalent in systems of weakly interactingWeak interactionWeak interaction , is one of the four fundamental forces of nature, alongside the strong nuclear force, electromagnetism, and gravity. It is responsible for the radioactive decay of subatomic particles and initiates the process known as hydrogen fusion in stars...
particles - from an atom (many-body assembly) to statistical ensembles of molecules (viscous fluids, amorphous solids, liquid crystals etc.) at molecular levels or as a whole (see "metastable phases of matter" and "grain piles" below). The abundance of states is more prevalent as the systems grow larger and/or if the forces of their mutual interaction are spatially less uniform or more diverse. - In dynamic systems (with feedbackFeedbackFeedback describes the situation when output from an event or phenomenon in the past will influence an occurrence or occurrences of the same Feedback describes the situation when output from (or information about the result of) an event or phenomenon in the past will influence an occurrence or...
) like electronic circuits, signal trafficking, decisional systems and neuroscience - it is the time-invariance of the active or reactive patterns with respect to the external influences that defines stability and metastability (see "brain metastability" below). Here the equivalent of the thermal fluctuations is the "white noise" affecting the signal propagation and the decision-making.
Quantum mechanics
Aggregated systems of subatomic particleSubatomic particle
In physics or chemistry, subatomic particles are the smaller particles composing nucleons and atoms. There are two types of subatomic particles: elementary particles, which are not made of other particles, and composite particles...
s—as described by quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
(quarks inside the nucleons and nucleons inside the atomic nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
, electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
ic systems of atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s inside molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s or atomic clusters)—are found to have many (a set of) distinguishable states of which only one is indefinitely stable: the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
or global minimum.
All other states besides the 'ground state' have higher, 'excited' energies. Of all these other states, metastable are the ones having lifetimes
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
lasting at least 102-103 times longer than the shortest lived states of the set.
A metastable state is then long lived (locally stable
Chemical stability
Chemical stability when used in the technical sense in chemistry, means thermodynamic stability of a chemical system.Thermodynamic stability occurs when a system is in its lowest energy state, or chemical equilibrium with its environment. This may be a dynamic equilibrium, where individual atoms...
with respect to configurations of 'neighbouring' energies) but not eternal (as the global minimum
Maxima and minima
In mathematics, the maximum and minimum of a function, known collectively as extrema , are the largest and smallest value that the function takes at a point either within a given neighborhood or on the function domain in its entirety .More generally, the...
is). Being 'excited' - of an energy value above the 'ground' - it will have to eventually 'de-excite'. Indeed, above the absolute 0 K
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
temperature, all system's states, metastable or not, have a non-zero probability to eventually 'decay' or spontaneously fall onto the ground state via tunnelling
Quantum tunnelling
Quantum tunnelling refers to the quantum mechanical phenomenon where a particle tunnels through a barrier that it classically could not surmount. This plays an essential role in several physical phenomena, such as the nuclear fusion that occurs in main sequence stars like the sun, and has important...
due to internal thermal fluctuations (in 'position' and 'velocity').
Nuclear physics
Some energetic states of an atomic nucleusAtomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
(having distinct spatial mass, charge, spin, isospin distributions) are much longer-lived than others (nuclear isomers of the same isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
).
Atomic and molecular physics
Some atomic energy levels are metastable. Transitions from these levels are typically those "forbidden" by electric dipole selection ruleSelection rule
In physics and chemistry a selection rule, or transition rule, formally constrains the possible transitions of a system from one state to another. Selection rules have been derived for electronic, vibrational, and rotational transitions...
s. This means that any transitions from this level are relatively unlikely to occur. In a sense, an electron that happens to find itself in a metastable configuration is trapped there. Of course, since transitions away from a metastable state are not impossible (merely unlikely), the electron will eventually be able to decay to a less energetic state by spontaneous emission. This property is made use of in lasers.
When light of suitable wavelength falls on atoms, their electrons jump to a higher energy state. When the incoming radiations are removed, the excited electron goes back to its original level within a duration of 10−8 seconds. However, when an electron goes to a metastable state, it remains there for a relatively longer duration of 10−3 seconds. This phenomenon leads to accumulation of electrons in the metastable state, since the rate of addition of electrons to the metastable state is higher than the rate of their de-excitation. This leads to the phenomenon called population inversion
Population inversion
In physics, specifically statistical mechanics, a population inversion occurs when a system exists in state with more members in an excited state than in lower energy states...
, which forms the basis of lasing action of lasers.
Chemistry
In chemical systems, a system of atoms or molecules involving a change in chemical bondChemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
can be in a metastable state, which lasts for a relatively long period of time. Molecular vibrations due to heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
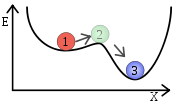
make chemical species at the energetic equivalent of the top of a round hill very short-lived. Metastable states that persist for many seconds (or years) are found in energetic valleys which are not the lowest possible valley (point 1 in illustration).
The stability or metastability of a given molecule depends in part on its environment, including temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, and the presence of catalyst
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
s or seed crystal
Seed crystal
A seed crystal is a small piece of single crystal/polycrystal material from which a large crystal of the same material typically is to be grown...
s (in the case of a solid state
Solid-state chemistry
Solid-state chemistry, also sometimes referred to as materials chemistry, is the study of the synthesis, structure, and properties of solid phase materials, particularly, but not necessarily exclusively of, non-molecular solids...
system). The presence of a liquid layer can help facilitate transitions between crystal states. The difference between producing a stable vs. metastable entity can have important consequences. For instances, having the wrong crystal polymorph
Polymorphism (materials science)
Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
can result in failure of a drug while in storage between manufacture and administration.
Reaction intermediate
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
s are very short-lived, and are actually thermodynamically unstable rather than "metastable". The IUPAC
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in individual countries. It is a member of the International Council for Science . The international headquarters of IUPAC is located in Zürich,...
recommends referring to these as "transient" rather than "metastable".
Metastability is also used to refer to specific situations in mass spectrometry and spectrochemistry.
Electron systems in biochemistry
The evolution of many-body quantum system between its characteristic set of states may be influenced by the following external actions:- The environment may act chaoticallyChaos theoryChaos theory is a field of study in mathematics, with applications in several disciplines including physics, economics, biology, and philosophy. Chaos theory studies the behavior of dynamical systems that are highly sensitive to initial conditions, an effect which is popularly referred to as the...
onto the system and add uncertainty to all state energies (while decreasing their lifetimes) — as in the spectral line broadening. - Just as well, 'resonantResonanceIn physics, resonance is the tendency of a system to oscillate at a greater amplitude at some frequencies than at others. These are known as the system's resonant frequencies...
' exterior actions may 'nudge' the system into a lower cohesive energy state while making it release an intrinsic amount or quantaQuantumIn physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete...
of its energy — as in the stimulated emissionStimulated emissionIn optics, stimulated emission is the process by which an atomic electron interacting with an electromagnetic wave of a certain frequency may drop to a lower energy level, transferring its energy to that field. A photon created in this manner has the same phase, frequency, polarization, and...
s. - Alternatively, external catalytic fields of forces may briefly flatten some of the barriers (ridges separating adjacent valleys) in the potentialPotential*In linguistics, the potential mood*The mathematical study of potentials is known as potential theory; it is the study of harmonic functions on manifolds...
landscape of the system and help it tunnel through to lower energy states (see image above). - Last but not least, under the impact of thermal or directional external actions, some systems (see macromoleculeMacromoleculeA macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
complexes involving enzymeEnzymeEnzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
-cofactorCofactor (biochemistry)A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
association) may wander for extremely long periods of time among a certain sub-group of their states (all having distinct configurations but energy differences within the thermal fluctuation range). As such the enzymes will enter a biochemical reaction sequence with an initial configuration, perform through its many steps as catalysts while continuously contorting, and eventually leave that reaction sequence in the same configuration as they have entered it, ready to perform again.
Statistical physics and thermodynamics
Non-equilibrium thermodynamicsNon-equilibrium thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with systems that are not in thermodynamic equilibrium. Most systems found in nature are not in thermodynamic equilibrium; for they are changing or can be triggered to change over time, and are continuously and discontinuously...
is a branch of physics that studies the dynamics of statistical ensembles of molecules via unstable states. Being "stuck" in a thermodynamic trough without being at the lowest energy state is known as being "kinetically persistent". The particular "motion" or "kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
" of the atoms involved has resulted in getting "stuck", despite there being preferable (lower-energy) alternatives.
Phases of matter
Metastable phases of matterPhase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
range from melting solids (or freezing liquids), boiling liquids (or condensing gasses) and sublimating solids
Sublimation (physics)
Sublimation is the process of transition of a substance from the solid phase to the gas phase without passing through an intermediate liquid phase...
to supercooled
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
liquids or superheated
Superheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
liquid-gas mixtures. Extremely pure, supercooled water stays liquid below 0 °C and remains so until applied vibrations or condensing seed doping will initiate crystallization
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
centers.
Condensed matter and macromolecules
In solid state physics, diamondDiamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
is a metastable form of carbon at standard temperature and pressure
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
. It can be converted to graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
(plus leftover kinetic energy), but only after overcoming an activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
- an intervening hill. Martensite
Martensite
Martensite, named after the German metallurgist Adolf Martens , most commonly refers to a very hard form of steel crystalline structure, but it can also refer to any crystal structure that is formed by displacive transformation. It includes a class of hard minerals occurring as lath- or...
is a metastable phase used to control the hardness of most steel. The bonds between the building blocks of polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s such as DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
, RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
and protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s are also metastable.

Sand
Sand is a naturally occurring granular material composed of finely divided rock and mineral particles.The composition of sand is highly variable, depending on the local rock sources and conditions, but the most common constituent of sand in inland continental settings and non-tropical coastal...
s form a pile due to friction
Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and/or material elements sliding against each other. There are several types of friction:...
. It is possible for an entire large sand pile to reach a point where it is stable, but the addition of a single grain causes large parts of it to collapse.
The avalanche
Avalanche
An avalanche is a sudden rapid flow of snow down a slope, occurring when either natural triggers or human activity causes a critical escalating transition from the slow equilibrium evolution of the snow pack. Typically occurring in mountainous terrain, an avalanche can mix air and water with the...
is a well-known problem with large piles of snow and ice crystals on steep slopes. In dry conditions, snow slopes act similarly to sandpiles. An entire mountainside of snow can suddenly slide due to the presence of a skier, or even a loud noise or vibration.
Electronic circuits
Metastability in electronicsMetastability in electronics
Metastability in electronics is the ability of a digital electronic system to persist for an unbounded time in an unstable equilibrium or metastable state....
is usually seen as a problem. A changing circuit is supposed to "settle" into one of a small number of desired states, but if the circuit is vulnerable to metastability, it can get "stuck" in an undesirable state.
Computational neuroscience
Metastability in the brainMetastability in the brain
In the field of computational neuroscience, the theory of metastability refers to the human brain’s ability to integrate several functional parts and to produce neural oscillations in a cooperative and coordinated manner, providing the basis for conscious activity.Metastability, a state in which...
is a phenomenon which is being studied in computational neuroscience
Computational neuroscience
Computational neuroscience is the study of brain function in terms of the information processing properties of the structures that make up the nervous system...
to elucidate how the human mind recognizes patterns. The term "metastability" here is used rather loosely. There is no "lower energy" state, but there are semi-transient signals in the brain which persist for a while and are different than the usual equilibrium state.

