
Cucurbituril
Encyclopedia
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
-linked macrocyclic molecules
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
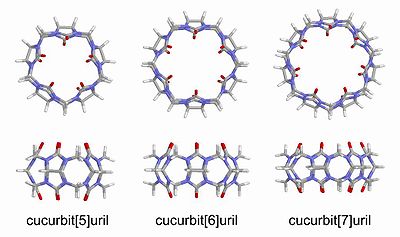
made of glycoluril [=C4H2N4O2=] monomers. The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity. The name is derived from the resemblance of this molecule with a pumpkin
Pumpkin
A pumpkin is a gourd-like squash of the genus Cucurbita and the family Cucurbitaceae . It commonly refers to cultivars of any one of the species Cucurbita pepo, Cucurbita mixta, Cucurbita maxima, and Cucurbita moschata, and is native to North America...
of the family of Cucurbitaceae
Cucurbitaceae
The plant family Cucurbitaceae consists of various squashes, melons, and gourds, including crops such as cucumber, pumpkins, luffas, and watermelons...
.
Cucurbiturils are commonly written as cucurbit[n]uril, where n is the number of glycoluril units. Two common abbreviations are CB[n], or simply CBn.
These compounds are particularly interesting to chemists because they are suitable hosts for an array of neutral and cationic species. The binding mode is thought to occur through hydrophobic interactions, and, in the case of cationic guests, through cation-dipole interactions as well. The dimensions of cucurbiturils are generally on the ~ 10 Å size scale. For instance, the cavity of cucurbit[6]uril has a height ~ 9.1 Å, an outer diameter ~ 5.8 Å, and an inner diameter ~ 3.9 Å.
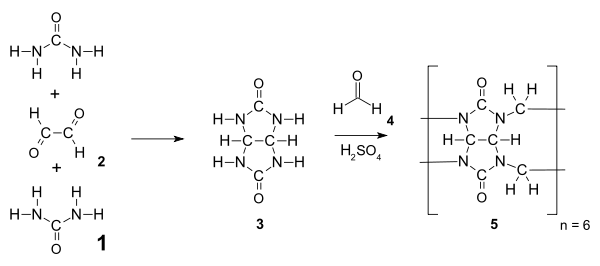
Cucurbiturils were first synthesized in 1905 by Behrend, by condensing glycoluril with formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
, but their structure was not elucidated until 1981. To date cucurbiturils composed of 5, 6, 7, 8, and 10 repeat units have all been isolated, which have internal cavity volumes of 82, 164, 279, 479, and 870 Å3 respectively. A cucurbituril composed of 9 repeat units has yet to be isolated (as of 2009). Other common molecular capsules that share a similar molecular shape with cucurbiturils include cyclodextrins, calixarene
Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde. The word calixarene is derived from calix or chalice because this type of molecule resembles a vase and from the word arene that refers to the aromatic building block...
s, and pillararene
Pillararene
Pillararenes are macrocycles composed of hydroquinone units linked in the para position. They are structurally similar to the cucurbiturils and calixarenes that play an important part in host-guest chemistry. arene], the first pillarene, was synthesized by Tomoki Ogoshi at Kanazawa University....
s.
Synthesis
Aminal
An aminal or aminoacetal is a functional group or type of chemical compound that has two amine groups attached to the same carbon atom: -C-. Again R can be hydrogen or an alkyl group....
s and synthesized from urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
1 and a diketone
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
(e.g., glyoxal
Glyoxal
Glyoxal is an organic compound with the formula OCHCHO. This yellow colored liquid is the smallest dialdehyde . Its tautomer acetylenediol is unstable.-Production:...
2) via a nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
to give the intermediate glycoluril 3. This intermediate is condensed
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
with formaldehyde to give hexamer
Hexamer
A hexamer is a thing composed out of six sub-units.In microbiology, a hexamer is one of the proteins composing the polyhedral protein shell that encloses the bacterial micro-compartments known as carboxysomes....
cucurbit[6]uril above 110 °C. Ordinarily, multifunctional monomers such as 3 would undergo a step-growth polymerization
Step-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth...
that would give a distribution of products, but due to favorable strain
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional...
and an abundance of hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing, the hexamer is the only reaction product isolated after precipitation.
Decreasing the temperature of the reaction to between 75 and 90 °C can be used to access other sizes of cucurbiturils including CB[5], CB[7], CB[8], CB[9], and CB[10]. CB[6] is still the major product; the other ring sizes are formed in smaller yields. The isolation of sizes other than CB[6] requires fractional crystallization and dissolution. CB[5], CB[6], CB[7], and CB[8] are all currently commercially available. The larger sizes are a particularly active area of research since they can bind larger and more interesting guest molecules, thus expanding their potential applications.
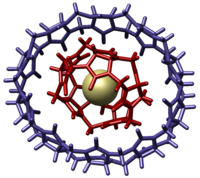
Inclusion compound
In host-guest chemistry an inclusion compound is a complex in which one chemical compound forms a cavity in which molecules of a second "guest" compound are located. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which...
containing CB[5] by fractional crystallization of the cucurbituril reaction mixture. The CB[10]•CB[5] was unambiguously identified by single crystal X-ray structural analysis that revealed the complex resembled a molecular gyroscope
Gyroscope
A gyroscope is a device for measuring or maintaining orientation, based on the principles of angular momentum. In essence, a mechanical gyroscope is a spinning wheel or disk whose axle is free to take any orientation...
. In this case the free rotation of the CB[5] within the CB[10] cavity mimics the independent rotation of a flywheel within the frame of a gyroscope.
Isolation of pure CB[10] could not be accomplished by direct separation methods since the compound has such a high affinity for CB[5]. The strong binding affinity for the CB[5] can be understood since it has a complementary size and shape to the cavity of the CB[10]. Pure CB[10] was isolated by Isaacs and coworkers in 2005 by introducing a more strongly binding melamine
Melamine
Melamine is an organic base and a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass and, if mixed with resins, has fire retardant properties due to its release of nitrogen gas when burned or charred, and has several other industrial uses....
diamine guest that is capable of displacing the CB[5]. The melamine diamine guest was then separated from the CB[10] by reaction with acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
that converted the positively charged amine groups to neutrally charged amides. Cucurbiturils strongly bind cationic guests, but by removing the positive charge from the melamine diamine guest reduces the association constant to the point it can be removed by washing with methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, and water. The CB[10] has an unusually large cavity (870 Å3) that's free and capable of binding extraordinarily large guests including a cationic calix[4]arene
Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde. The word calixarene is derived from calix or chalice because this type of molecule resembles a vase and from the word arene that refers to the aromatic building block...
.
Applications
Cucurbiturils have been used by chemists for various applications, including drug delivery, asymmetric synthesis, molecular switching, and dye tuning.Supramolecular host molecules
Molecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, electrostatic and/or electromagnetic effects...
and have a particularly high affinity for positively charged or cationic compounds. High association constants with positively charged molecules are attributed to the carbonyl groups that line each end of the cavity and can interact with cations in a similar fashion to crown ethers. The affinity of cucurbiturils can be very high. For example the affinity equilibrium constant of cururbit[7]uril with the positively charged 1-aminoadamantane hydrochloride is experimentally determined at 4.23*1012.
Host guest interactions also significantly influence solubility behavior of cucurbiturils. Cucurbit[6]uril dissolves poorly in just about any solvent but solubility is greatly improved in a solution of potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
or in an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic solution. The cavitand
Cavitand
A cavitand is a container shaped molecule. The cavity of the cavitand allows it to engage in host-guest chemistry with guest molecules of a complementary shape and size. Examples include cyclodextrins, calixarenes, pillararenes and cucurbiturils....
forms a positively charged inclusion compound
Inclusion compound
In host-guest chemistry an inclusion compound is a complex in which one chemical compound forms a cavity in which molecules of a second "guest" compound are located. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which...
with a potassium ion or a hydronium ion respectively which have much greater solubility that the uncomplexed neutral molecule.
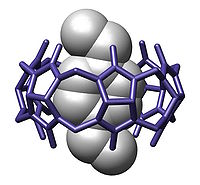
CB[10] is large enough to hold other molecular hosts such as a calixarene
Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde. The word calixarene is derived from calix or chalice because this type of molecule resembles a vase and from the word arene that refers to the aromatic building block...
molecule. With a calixarene guest different chemical conformations (cone, 1,2-alternate, 1,3-alternate) are in rapid equilibrium. Allosteric control is provided when an adamantane
Adamantane
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
molecule forces a cone conformation with a calixarene - adamantane inclusion complex within a CB[10] molecule.
Rotaxane macrocycles
Given their high affinities to form inclusion complexes cucurbiturils have been employed as the macrocycleMacrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
s component of a rotaxane
Rotaxane
A rotaxane is a mechanically-interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle" . The name is derived from the Latin for wheel and axle...
. After formation of the supramolecular assembly
Supramolecular assembly
A supramolecular assembly or "supermolecule" is a well defined complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules , it is more often used to denote larger complexes of molecules that form sphere-, rod-, or sheet-like...
or threaded complex with a guest molecule such as hexamethylene diamine the two ends of the guest can be reacted with bulky groups that will then act as a stoppers preventing the two separate molecules from dissociating.
In another rotaxane system with a CB[7] wheel, the axle is a 4,4'-bipyridinium or viologen
Viologen
Viologens are toxic bipyridinium derivatives of 4,4'-bipyridyl. The name is because this class of compounds is easily reduced to the radical mono cation, which is intensely blue coloured....
subunit with two carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
terminated aliphatic N-substituents at both ends. In water at concentration higher than 0.5 M complexation is quantitative without need of stoppers. At pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
= 2 the carboxylic end-group
End-group
An end-group in polymer chemistry is a constitutional unit that is an extremity of a macromolecule or oligomer molecule. For example the end-group of a PET polyester may be an alcohol group or a carboxylic acid group...
s are protonated and the wheel shuttles back and forth between them as evidenced by the presence of just two aromatic viologen protons in the proton NMR
Proton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
spectrum. At pH = 9 the wheel is locked around the viologen center.
Drug delivery vehicles
Cucurbituril's host-guest properties have been explored for drug delivery vehicles. The potential of this application has been explored with cucurbit[7]uril that forms an inclusion compoundInclusion compound
In host-guest chemistry an inclusion compound is a complex in which one chemical compound forms a cavity in which molecules of a second "guest" compound are located. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which...
with the important cancer fighting drug oxaliplatin
Oxaliplatin
Oxaliplatin is a coordination complex that is used in cancer chemotherapy. These platinum-based drugs are usually classified as alkylating agents, although they are not actually alkylating groups ....
. CB[7] was employed despite the fact that it is more difficult to isolate since it has much greater solubility in water and its larger cavity size can accommodate the drug molecule. The resulting complex was found to have increased stability and greater selectivity that may lead to less side effects.
Supramolecular catalysts
Cucurbiturils have also been explored as supramolecular catalysts. Larger cucurbiturils, such as cucurbit[8]uril can bind multiple guest molecules. CB[8] forms a complex 2:1 (guest:host) with (E)-diaminostilbene dihydrochloride which is accommodated by CB[8]’s larger internal diameter of 8.8 angstromÅngström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
and height 9.1 angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
. The close proximity and optimal orientation of the guest molecules within the cavity enhances the rate of the photochemical
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
cyclization to give cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
dimer with a 19:1 stereoselectivity for the syn configuration when bound to CB[8]. In the absence of CB[8] the cyclization reaction does not occur, but only the isomerization of the trans isomer to the cis isomer is observed.
Dye Tuning
The dye-tuning capabilities cucurbiturils have been explored by researchers in recent years. In general, it has been found that the confined, low-polarity environment provided by the cucurbiturils leads to enhanced brightness, increased photostability, increased fluorescence lifetimes, and solvatochromism consistent with moving to an environment of lower polarity.Related compounds
Inverted cucurbiturils or iCB[x] are CB analogues with one glycoluril repeating unit inverted. In this unit the methineMethine
In chemistry, methine is a trivalent functional group CH, derived formally from methane. The methine group consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen...
protons actually point into the cavity and this makes the cavity less spacious. Inverted cucurbiturils form as a side-product in CB-forming reactions, with yields between 2 and 0.4%. Isolation of this type of CB compound is possible because it is more difficult to form inclusion compounds that ordinarily form with regular CB's. Inverted cucurbiturils are believed to be the kinetically controlled reaction products because the addition of deuterated
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
or DCl to iCB[6] results in a mixture of CB[5], CB[6] and CB[7]in a 24:13:1 ratio.
A cucurbituril cut in half along the equator is called a hemicucurbituril
Hemicucurbituril
A hemicucurbituril is a macrocycle composed of alternating units of methylene and N-substituted ethylene urea units. Hemicucurbit[6]uril is a hexamer. This compound closely resembles cucurbituril cut in half along the equator and the chemistry is also similar. The ethylene urea units also...
.

