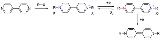
Viologen
Encyclopedia
Viologens are toxic bipyridinium
derivatives of 4,4'-bipyridyl. The name is because this class of compounds is easily reduced to the radical mono cation
, which is intensely blue coloured.
Possibly the best known viologen is paraquat
, which is one of the world's most widely used herbicide
s.
and oxidation.
In an experimental electrolysis
setup, viologen in solution with sodium sulfate
can be reduced at the cathode
with simultaneous formation of hydrogen gas. Oxygen generated at the anode
is capable of oxidizing the radical ion back to the viologen.
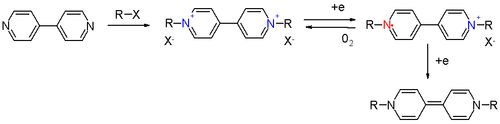 Further reduction yields a yellow quinoid
Further reduction yields a yellow quinoid
compound. Diquaternary derivatives of 2,2'-bipyridyl give a green radical anion.
In extended viologens conjugated
oligomer
s such as based on aryl
, ethylene
and thiophene
units are inserted between the pyridine
units. The bipolaron
di-octyl bis(4-pyridyl)biphenyl viologen 2 in scheme 2 can be reduced by sodium amalgam
in DMF
to the neutral viologen 3.
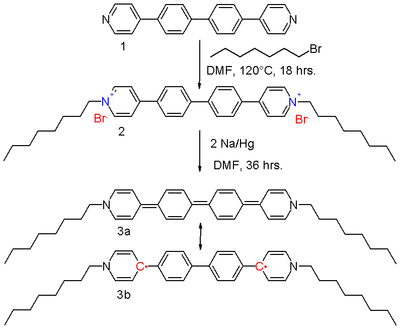 The resonance structures of the quinoid 3a and the biradical 3b contribute equally to the hybrid structure. The driving force for the contributing 3b is the restoration of aromaticity
The resonance structures of the quinoid 3a and the biradical 3b contribute equally to the hybrid structure. The driving force for the contributing 3b is the restoration of aromaticity
with the biphenyl
unit. From X-ray crystallography
it is established that the molecule is effectively coplanar with slight nitrogen pyramidalization, and that the central carbon bonds are longer (144 pm) than what would be expected for a double bond
(136 pm). Further research shows that the diradical exists as a mixture of triplet
s and singlet
s although remarkably an ESR signal is absent. In this sense the molecule resembles Tschischibabin's hydrocarbon
discovered in 1907. It also shares with this molecule a blue color in solution, and a metallic green color as crystals.
Compound 3 is a very strong reducing agent
with a redox potential of - 1.48 V again because aromaticity is restored. The compound is also a liquid crystal
with multiple liquid crystal phases in the melt as a result of the molecule's structure with a flat and rigid core and flexible linear alkyl arms.
Viologen catalysts have been reported to have the potential to catalytically oxidize glucose
and other carbohydrate
s in a mildly alkaline solution, which makes direct carbohydrate fuel cell
s possible.
Pyridinium
Pyridinium refers to the cationic form of pyridine. This can either be due to protonation of the ring nitrogen or because of addition of a substituent to the ring nitrogen, typically via alkylation. The lone pair of electrons on the nitrogen atom of pyridine is not delocalized, and thus pyridine...
derivatives of 4,4'-bipyridyl. The name is because this class of compounds is easily reduced to the radical mono cation
Radical ion
A radical ion is a free radical species that carries a charge. Radical ions are encountered in organic chemistry as reactive intermediates and in mass spectrometry as gas phase ions...
, which is intensely blue coloured.
Possibly the best known viologen is paraquat
Paraquat
Paraquat is the trade name for N,N′-dimethyl-4,4′-bipyridinium dichloride, one of the most widely used herbicides in the world. Paraquat, a viologen, is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic to human beings and animals...
, which is one of the world's most widely used herbicide
Herbicide
Herbicides, also commonly known as weedkillers, are pesticides used to kill unwanted plants. Selective herbicides kill specific targets while leaving the desired crop relatively unharmed. Some of these act by interfering with the growth of the weed and are often synthetic "imitations" of plant...
s.
Electrochemistry
Viologens are also investigated for use in electrochromic systems because of their ability to change color reversibly many times upon reductionRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
and oxidation.
In an experimental electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
setup, viologen in solution with sodium sulfate
Sodium sulfate
Sodium sulfate is the sodium salt of sulfuric acid. When anhydrous, it is a white crystalline solid of formula Na2SO4 known as the mineral thenardite; the decahydrate Na2SO4·10H2O has been known as Glauber's salt or, historically, sal mirabilis since the 17th century. Another solid is the...
can be reduced at the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
with simultaneous formation of hydrogen gas. Oxygen generated at the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
is capable of oxidizing the radical ion back to the viologen.

Quinone
A quinone is a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C– groups with any necessary rearrangement of double bonds," resulting in "a fully conjugated cyclic dione structure."...
compound. Diquaternary derivatives of 2,2'-bipyridyl give a green radical anion.
In extended viologens conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
oligomer
Oligomer
In chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
s such as based on aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
, ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
and thiophene
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
units are inserted between the pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
units. The bipolaron
Bipolaron
- Bipolarons in physics :In physics, a bipolaron is a bound pair of two polarons. An electron in a material may cause a distortion in the underlying lattice. The combination of electron and distortion is known as a polaron...
di-octyl bis(4-pyridyl)biphenyl viologen 2 in scheme 2 can be reduced by sodium amalgam
Sodium amalgam
Sodium amalgam, commonly denoted Na, is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as strong reducing agents with better handling properties compared...
in DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
to the neutral viologen 3.

Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
with the biphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
unit. From X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
it is established that the molecule is effectively coplanar with slight nitrogen pyramidalization, and that the central carbon bonds are longer (144 pm) than what would be expected for a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
(136 pm). Further research shows that the diradical exists as a mixture of triplet
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
s and singlet
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
s although remarkably an ESR signal is absent. In this sense the molecule resembles Tschischibabin's hydrocarbon
Non-Kekulé molecule
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned classical Kekulé structures. Since non-Kekulé molecules have two or more formal radical centers, their spin-spin interactions can cause electrical conductivity or ferromagnetism , and applications to functional materials are...
discovered in 1907. It also shares with this molecule a blue color in solution, and a metallic green color as crystals.
Compound 3 is a very strong reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
with a redox potential of - 1.48 V again because aromaticity is restored. The compound is also a liquid crystal
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
with multiple liquid crystal phases in the melt as a result of the molecule's structure with a flat and rigid core and flexible linear alkyl arms.
Viologen catalysts have been reported to have the potential to catalytically oxidize glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and other carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s in a mildly alkaline solution, which makes direct carbohydrate fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s possible.
External links
- Experimental details of viologen electrolysis from the University of RegensburgUniversity of RegensburgThe University of Regensburg is a public research university located in the medieval city of Regensburg, Bavaria, a city that is listed as a UNESCO World Heritage Site. The university was founded on July 18, 1962 by the Landtag of Bavaria as the fourth full-fledged university in Bavaria...

