Takai olefination
Encyclopedia
Takai olefination in organic chemistry
describes the organic reaction
of an aldehyde
with a diorganochromium compound to form an alkene
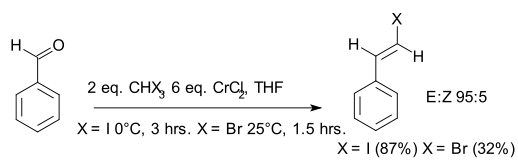
. In the original 1986 publication the aldehyde is benzaldehyde
and the organochromium species is generated from iodoform
or bromoform
and an excess of chromium(II) chloride
. The reaction product is a vinyl halide
. The main selling point is the E-configuration of the double bond. According to the principal investigator Kazuhiko Takai, existing alternatives such as the Wittig reaction
only yield mixtures.
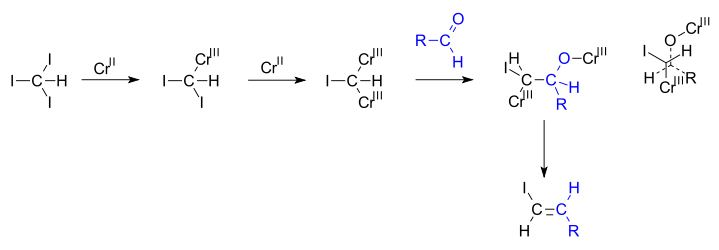
In the reaction mechanism
proposed by Takai, chromium(II) is oxidized to chromium(III) when replacing both halogen atoms. The geminal carbodianion complex thus formed reacts with the aldehyde in a 1,2-addition along one of the carbon to chromium bonds and in the next step both chromium bearing groups engage in an elimination reaction
. In newman projection
it can be seen how the steric bulks of chromium groups and the steric bulks of the alkyl and halogen groups drive this reaction towards anti elimination .
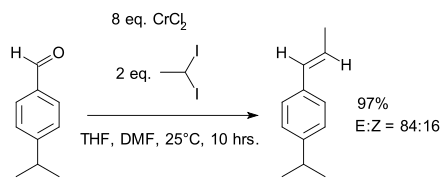
In a second publication the scope of the reaction was extended to diorganochromium intermediates bearing alkyl groups instead of halogens :
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
describes the organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
with a diorganochromium compound to form an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. In the original 1986 publication the aldehyde is benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
and the organochromium species is generated from iodoform
Iodoform
Iodoform is the organoiodine compound with the formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating odor and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant...
or bromoform
Bromoform
Bromoform is a pale yellowish liquid with a sweet odor similar to chloroform, a halomethane or haloform. Its refractive index is 1.595 . Bromoform is produced naturally by phytoplankton and seaweeds in the ocean and this is thought to be the predominant source to the environment...
and an excess of chromium(II) chloride
Chromium(II) chloride
Chromium chloride is the chemical compound with the formula CrCl2. This white, crystalline solid is used for the synthesis of other chromium complexes. CrCl2 is hygroscopic. It dissolves in water to give bright blue solutions that are easily oxidized by air to give Cr-containing products...
. The reaction product is a vinyl halide
Vinyl halide
In organic chemistry, a vinyl halide is any alkene with at least one halide substituent bonded directly on one of the unsaturated carbons. Vinyl chloride is one such substance....
. The main selling point is the E-configuration of the double bond. According to the principal investigator Kazuhiko Takai, existing alternatives such as the Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
only yield mixtures.
In the reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
proposed by Takai, chromium(II) is oxidized to chromium(III) when replacing both halogen atoms. The geminal carbodianion complex thus formed reacts with the aldehyde in a 1,2-addition along one of the carbon to chromium bonds and in the next step both chromium bearing groups engage in an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
. In newman projection
Newman projection
A Newman projection, useful in alkane stereochemistry, visualizes chemical conformations of a carbon-carbon chemical bond from front to back, with the front carbon represented by a dot and the back carbon as a circle . The front carbon atom is called proximal, while the back atom is called distal...
it can be seen how the steric bulks of chromium groups and the steric bulks of the alkyl and halogen groups drive this reaction towards anti elimination .
In a second publication the scope of the reaction was extended to diorganochromium intermediates bearing alkyl groups instead of halogens :