
Ullmann condensation
Encyclopedia
The Ullmann condensation or Ullmann ether synthesis is a variation of the Ullmann reaction
, in which a phenol
is coupled to an aryl
halide
to create a diaryl ether in the presence of a copper
compound, named after Fritz Ullmann
. The corresponding aniline
or aryl amide
reaction is sometimes called Goldberg reaction, named after Irma Goldberg.
An example is the synthesis of p-nitrophenyl phenyl ether
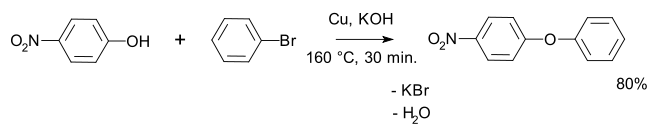 An active copper powder that is required for this reaction can be prepared by the reduction
An active copper powder that is required for this reaction can be prepared by the reduction
of copper sulfate by zinc
metal in hot water causing the precipitation
of elementary copper.
The reaction often requires high-boiling polar solvents such as N-methylpyrrolidone, nitrobenzene
or dimethylformamide
and high temperatures (often in excess of 210°C) with stochiometric amounts of copper. The aryl halide is activated by electron-withdrawing groups or carries a carboxylic acid
group in the aromatic ortho position. The research field was revitalized with the introduction of catalytic copper reactions in 2001 -2003 using up to 0.1 equivalent copper iodide, base and a diamine ligand.
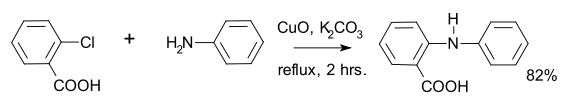
, an intermediate of acridone
via the Goldberg reaction:
 An Ullmann-type aromatic amination reaction between an aryl iodide and an aryl amine as coupling partners has been published. The catalyst used is formed from copper(I) iodide
An Ullmann-type aromatic amination reaction between an aryl iodide and an aryl amine as coupling partners has been published. The catalyst used is formed from copper(I) iodide
and phenanthroline
. As this reaction proceeds well with an electron-rich aryl iodide it is a valuable alternative to the Buchwald-Hartwig amination reaction
, which gives best yields with electron-poor aryl halides.
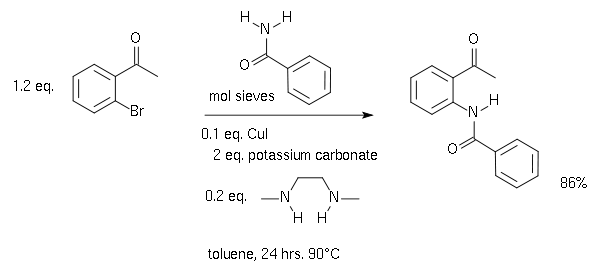
The scope is extended to amide
s for example in the synthesis of this Camps cyclization precursor :
in the Hurtley reaction.
In the original scope the arene was 2-bromobenzoic acid, the carbon nucleophile a malonic ester and other dicarbonyl compounds and the base sodium ethoxide
.
Ullmann reaction
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides with copper. The reaction is named after Fritz Ullmann....
, in which a phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
is coupled to an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
to create a diaryl ether in the presence of a copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
compound, named after Fritz Ullmann
Fritz Ullmann
Fritz Ullmann was a German chemist.Ullmann was born in Fürth and started studying chemistry in Nuremberg, but received his PhD of the University of Geneva for work with Carl Gräbe in 1895...
. The corresponding aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
or aryl amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
reaction is sometimes called Goldberg reaction, named after Irma Goldberg.
An example is the synthesis of p-nitrophenyl phenyl ether

Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of copper sulfate by zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
metal in hot water causing the precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
of elementary copper.
The reaction often requires high-boiling polar solvents such as N-methylpyrrolidone, nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
or dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
and high temperatures (often in excess of 210°C) with stochiometric amounts of copper. The aryl halide is activated by electron-withdrawing groups or carries a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
group in the aromatic ortho position. The research field was revitalized with the introduction of catalytic copper reactions in 2001 -2003 using up to 0.1 equivalent copper iodide, base and a diamine ligand.
Goldberg reaction
An example of a Goldberg reaction is the synthesis of fenamic acidFenamic acid
Fenamic acid is a molecule which serves as a parent structure for several non-steroidal anti-inflammatory drugs, including mefenamic acid, tolfenamic acid, flufenamic acid, and meclofenamic acid....
, an intermediate of acridone
Acridone
Acridone is an organic compound based on the acridine skeleton, with a carbonyl group at the 9 position. It may be synthesized by the self-condensation of N-phenylanthranilic acid.-Derivatives:...
via the Goldberg reaction:

Copper(I) iodide
Copper iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding....
and phenanthroline
Phenanthroline
Phenanthroline is a heterocyclic organic compound. As a bidentate ligand in coordination chemistry, it forms strong complexes with most metal ions...
. As this reaction proceeds well with an electron-rich aryl iodide it is a valuable alternative to the Buchwald-Hartwig amination reaction
Buchwald-Hartwig reaction
The Buchwald–Hartwig reaction is a chemical reaction used in organic chemistry for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed cross-coupling of amines with aryl halides. Though publications with similar focus were published as early as 1983, credit for its development is...
, which gives best yields with electron-poor aryl halides.
The scope is extended to amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s for example in the synthesis of this Camps cyclization precursor :

Hurtley reaction
The nucleophile can also be carbon as in a carbanionCarbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
in the Hurtley reaction.
In the original scope the arene was 2-bromobenzoic acid, the carbon nucleophile a malonic ester and other dicarbonyl compounds and the base sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
.

