TGN1412
Encyclopedia
TGN1412 is the working name of an immunomodulator
y drug which was withdrawn from development after inducing severe inflammatory reactions in the first human subjects to receive the drug.
Originally intended for the treatment of B cell
chronic lymphocytic leukemia
(B-CLL) and rheumatoid arthritis
, it is a humanised monoclonal antibody
that not only binds to, but is a strong agonist
for, the CD28
receptor of the immune system
's T cell
s. CD28 is the co-receptor for the T cell receptor; It binds to receptors on the interacting partner in the reaction through one of its ligands (B7 family).
In its first human clinical trial
s in March 2006, it caused catastrophic systemic organ failure in the subjects, despite being administered at a supposed sub-clinical dose of 0.1 mg per kg; some 500 times lower than the dose found safe in animals. Six volunteers were hospitalized on 13 March 2006, at least four of these suffering from multiple organ dysfunction
, and one trial volunteer is said to show signs of developing cancer
.
The developing company, TeGenero Immuno Therapeutics
, entered into insolvency
proceedings later in 2006. Tentative opinions from an as-yet uncompleted inquiry suggest that the problems resulted from "unforeseen biological action in humans", rather than breach of trial protocols, and the case therefore has had important ramifications for future trials of potentially powerful clinical agents.
Scientists in early 2007 put forth the theory that the drug acted in a different fashion in humans as compared with the laboratory animals in which the drug was first tried. The severe reactions in humans could have only occurred, they believe, in animals with memory T lymphocytes. Animals raised in a sterile lab would presumably have no 'memory' of previous illnesses, thus would not exhibit the severe reactions that occurred in the human subjects. However this is a misunderstanding of the research: the research says that non-human animals studied have fewer memory T cells than humans, and that stimulation through the CD28 receptor alone in memory T cells causes them to infiltrate organs and also activates them. In conjunction with the probable differences between the animal CD28 and the human CD28 protein sequence, and the fact that the antibody had been 'humanized' so as to be ignored by the human immune system which may not be similarly applicable in the experimental animal systems, one would expect differences in response. The surprise to the authorities and the firm was perhaps, ironically, that the drug was too powerful, and by starting with a dose regime based on animal studies rather than employing a more 'homeopathic' regime, they brought the industry into disrepute.
The drug, which was designated as an orphan medical product
by the European Medicines Agency
in March 2005, was developed by TeGenero Immuno Therapeutics
, tested by Parexel
and manufactured by Boehringer-Ingelheim
. TeGenero announced the first elucidation of the molecular structure of CD28 almost exactly one year prior to commencement of the TGN1412 phase I clinical trial.
s of 5.11A1 were cloned into the framework of human IgG and combined with IgG1 (TGN1112) or IgG4 (TGN1412) constant regions. According to the company's Investigator Brochure, "TGN1412 is a humanised monoclonal antibody directed against the human CD28 antigen. The molecule was genetically engineered by transfer of the complementarity determining regions (CDRs) from heavy and light chain variable region sequences of a monoclonal mouse anti-humanC28 [sic] antibody (5.11A1, Luhder et al., 2003) into human heavy and light chain variable frameworks. Humanised variable regions were subsequently recombined with a human gene coding for the IgG4 gamma chain and with a human gene coding for a human kappa chain, respectively." The recombinant genes were transfected into Chinese hamster ovary cell
s and the recombinant antibody harvested from culture supernatant.
Swag
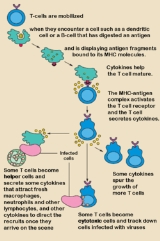
 Activation of T cells normally requires both engagement of the antigen receptor
Activation of T cells normally requires both engagement of the antigen receptor
(signal 1) and co-stimulation
(signal 2). Studies of monoclonal antibodies specific for mouse, rat, or human CD28 identified so-called "superagonistic" antibodies that could stimulate T cells without concurrent antigen-receptor stimulation (signal 1). Whether this activity represents a stronger activity or a different activity is uncertain. Two antibodies specific for human CD28 were identified. The more active of the two, TGN1112 (originally called 5.11A1), belonged to the IgG1 class of immunoglobulins. The other, TGN1412 (clone 9D7), belonged to the IgG4 class. The TCR-independent agonism of these antibodies involved binding to a specific part of the CD28 molecule called the C"D loop. It was initially hypothesized that an antibody with this property could be therapeutically useful in stimulating the immune system in immunosuppressed
patients. However, in vitro
and in vivo
data from animal studies later suggested that administration would lead to preferential activation of regulatory T cell
s, leading to a net effect of T-cell downregulation. On its website, the company writes: "A pronounced T-cell activation and expansion mediated by CD28-SuperMAB in animal models is accompanied by the expression of anti-inflammatory cytokine
s, like IL-10, rather than by the toxic cytokine storm
of pro-inflammatory mediators induced by other agents that address the TCR complex.". As it turned out, the results of the first trial in humans indicate that this may not always be the case.
A new explanation for the trial mishap was suggested by the findings of a recent paper in Clinical Immunology. Pillai et al. found that all T cells that get activated using conventional TCR-mediated stimulation become regulatory for a brief time and express FOXP3. However, eventually most of these cells downregulate their regulatory capabilities and become effector cells. Thus, attempts to induce FOXP3+ T cells might also induce effector cells capable of causing tissue damage.
Other cells activated by CD28 ligation in humans are eosinophil granulocyte
s. They can release IFN-γ, IL-2, IL-4, and IL-13. However, most in vitro experiments are limited to the use of purified peripheral blood mononuclear cells (PBMN's) that do not contain those cells.
To function as an agonist, it has been suggested that TGN1412 needs to be a whole antibody
, including the constant (Fc) region. According to a report by TeGenero, the F(ab)2 is not able to generate the required stimulation. Unlike the related clone TGN1112, an IgG1, TGN1412 is of the subclass IgG4. This choice was made as TGN1112 showed antibody-dependent cellular cytotoxicity on CD28+ Jurkat cells. Thus the function of antibody binding via an Fcγ receptor seems to be a requirement for the immune regulation. However, cell opsonisation by antibody leads normally to phagocytosis of the labeled cells, as seen in the case of HIV
.
s were conducted by Parexel at an independent clinical trials unit in leased space on the premises of Northwick Park and St. Mark's Hospital, London
, on 13 March 2006. Parexel is a company that carries out drug trials on behalf of pharmaceutical and biotechnology companies. Healthy volunteers were recruited to the study with a £2,000 fee, reportedly much higher than the 'few hundred quid' offered for other medical tests in the region. The trial resulted in hospitalization of all six volunteers administered the drug, at least four of whom suffered multiple organ dysfunction
.
The payment was seized upon by newspapers and reported in a sensationalist fashion. Good Clinical Practice (GCP) prohibits payments being made to Phase I trial volunteers on the basis of risk, and specifies that payments must be based upon the amount of time given up and the number of invasive procedures (e.g. blood sampling). For most trials, payments are in the range of around £1,000 per week - but the 'few hundred quid' trials mentioned above are those which the general public are most familiar with (since they only last a day or two, do not require working individuals to take time off work, and hence are more common).
The trial was a double-blind, randomized, placebo-controlled study
, with two of the eight subjects receiving a placebo
, and six receiving 1/500th of the highest dose used in previous experiments with cynomolgus macaques
. All six of the trial subjects who received the drug were male, aged 19 to 34 (median 29.5); none had a notable medical history, and all were well in the 2 weeks before the trial. The drug was given by intravenous infusion, starting at 8am, with an interval of around 10 minutes between patients, and each infusion lasting from 3 to 6 minutes. Roughly five minutes after the last participant had received his dose, the participant who had received the first dose complained of headache, and soon afterwards fever and pain. He took his shirt off, complaining that he felt like he was burning. Shortly after, the remaining participants who received the actual drug also became ill, vomiting and complaining of severe pain. The first patient was transferred to the Northwick Park hospital's intensive care unit 12 hours after infusion, with the others following within the next 4 hours. A severely affected volunteer, Mohammed Abdalla, a 28-year old who said he had hoped to set his brother up in business in Egypt, was described as having suffered a ballooned head. This led to his description as being similar to the "Elephant Man
".
All of the men were reported to have experienced cytokine release syndrome
resulting in angioedema
, swelling of skin
and mucous membrane
s, akin to the effects of the complement cascade in severe allergic reaction. The patients were treated with corticosteroid
s to reduce inflammation, and plasma-exchange
to attempt to remove TGN1412 from their circulation. The treating doctors confirmed in August 2006 that all six men had suffered from a cytokine storm
, and that, paradoxically, the men's white blood cells had vanished almost completely several hours after administration of TGN1412.
According to a press release from 5 July 2006 on the North West London Hospitals NHS Trust website, where the men were treated, the patients continued to improve and "five of them went home within a month of the incident, while one patient remained in hospital until 26 June, when he also went home." However, Head of pharmacology at University College London Trevor Smart has suggested that the men may never fully recover, and may suffer long-term disruption to their immune systems.
An article by The Sunday Times
on 30 July 2006 reported lawyers' claims that the long-term damage to the patients may be worse than originally thought. Medical assessment by immunologist Professor Richard Powell were said to have revealed that the blood of the patients contained a low number of regulatory T-cells, below one percent compared to three to five percent for healthy male adults - although the clinical significance of any such finding are unknown. Powell also reportedly claimed that one of the patients has "definite early signs that a lymphoid malignancy is developing". Some of the men involved in the trial are said to have been told that they face "a lifetime of contracting cancers and all the various auto-immune diseases from lupus
to MS
, from rheumatoid arthritis
to ME
."
TGN1412 had not previously been given to humans; however, the trial was preceded by animal testing, including in non-human primates
. The company claims that these did not indicate any safety issues. The US patent application states "it could be shown in a pilot study that an in vitro
administration of anti-human CD28-SuperMAB induces in a rhesus monkey in vivo
a profound activation of T cells, without clinically visible side effects" and goes on to say "This antibody—in spite of its strong T cell-stimulatory properties—is very well tolerated in vivo, in contrast to all other known T cell
activating substances."
TeGenero
has apologized to the families involved, insists that these effects were completely unexpected, and said that all protocols have been followed. An investigation by the UK drug regulator reported that the reaction was not due to contamination of the dose, or an incorrect dose being administered, but suggested that the problem was due to "on target" effects of the drug. Criticism has been raised that six participants were given the drug in such a short time, which is against the recommendations of standard literature. However, the Medicines and Healthcare products Regulatory Agency
(MHRA) has confirmed that they had approved the trial, including the protocol of giving the dose to all men within a short time. It appears the MHRA approved a protocol involving the doses being administrated between 8.00h-10.00h (i.e., 2 hours). One of the placebo-receiving participants has explained the doses were given with 2-minute intervals. Even though the participants were dosed with short intervals, this is not a deviation from the approved protocol.
The MHRA has further stated that the initial dose of TGN1412 was intended to be the first of a course of injections, with the dosage being ramped up over time. It has been reported that the initial dose was one five-hundredth of that which the animal studies indicated was a maximum safe dose. Dr. David Glover, an industry consultant, has suggested that because the antibody was raised against human CD28, the safe dosage may have been lower in humans than in animals.
Another issue is whether the company should have known the drug would provoke this reaction in humans. The comments on the company webpage and in the patent application at least indicate the company knew this type of drug could cause a cytokine storm
. An immunologist contacted by New Scientist
and who wished to be anonymous has commented that “You don’t need to be a rocket scientist to work out what will happen if you non-specifically activate every T cell in the body.”
While the drug had appeared to be safe in animal models, researchers have noted that there are reasons why these may not be indicative of the response in humans, particularly with respect to this type of drug. The BBC reported that “two of 20 monkeys used in earlier tests suffered an increase in the size of lymph node
s,” but that “this information was given to the men and submitted to the test regulators.” TeGenero say this was transient and was evidence of the extra T cells that the drug produces. Experiments with another drug affecting the CD28 receptor (but to a lesser extent than TGN1412) had also shown side effects in human trials. There have been criticisms that the risks taken and the design of the protocol were insufficiently justified by proper statistical evidence
.
German regulatory authorities inspected the production of the material by Boehringer Ingelheim, looking at the manufacture, testing, storage and distribution of the TGN1412. No deficiencies were identified which could have contributed to the serious adverse effects. Although tests are ongoing on the actual material used, the MHRA state that tests are consistent with the TGN1412 being up to specification at the moment.
The MHRA have concluded that the most likely cause of the reaction in trial subjects was an unpredicted biological action of the drug in humans. The interim report recognises that important scientific and medical questions about the risks of testing these agents in human subjects have been raised. To that end, the UK Secretary of State for Health has agreed to establish a group of leading international experts to consider those issues and to provide a report on the future authorisation of such trials (with an interim report at three months).
Until the expert group has reported, all further clinical trial applications involving first-in-humans trials of any monoclonal antibody or other novel molecules targeting the immune system will not be authorised in the UK, without having had additional expert opinion on whether the effects seen in the TGN1412 may be repeated. There will be a fuller report on the TGN1412 trial in future, but the expert group will run concurrently.
Immunomodulator
An immunomodulator, also known as an immunotherapy is a substance which has an effect on the immune system.- Immunosuppressants :Inhibits immune response in organ transplantation and autoimmune diseases.- Immunostimulants :...
y drug which was withdrawn from development after inducing severe inflammatory reactions in the first human subjects to receive the drug.
Originally intended for the treatment of B cell
B cell
B cells are lymphocytes that play a large role in the humoral immune response . The principal functions of B cells are to make antibodies against antigens, perform the role of antigen-presenting cells and eventually develop into memory B cells after activation by antigen interaction...
chronic lymphocytic leukemia
Chronic lymphocytic leukemia
B-cell chronic lymphocytic leukemia , also known as chronic lymphoid leukemia , is the most common type of leukemia. Leukemias are cancers of the white blood cells . CLL affects B cell lymphocytes. B cells originate in the bone marrow, develop in the lymph nodes, and normally fight infection by...
(B-CLL) and rheumatoid arthritis
Rheumatoid arthritis
Rheumatoid arthritis is a chronic, systemic inflammatory disorder that may affect many tissues and organs, but principally attacks synovial joints. The process produces an inflammatory response of the synovium secondary to hyperplasia of synovial cells, excess synovial fluid, and the development...
, it is a humanised monoclonal antibody
Humanized antibody
Humanized antibodies are antibodies from non-human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans. The process of "humanization" is usually applied to monoclonal antibodies developed for administration to humans...
that not only binds to, but is a strong agonist
Agonist
An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. Agonists often mimic the action of a naturally occurring substance...
for, the CD28
CD28
CD28 is one of the molecules expressed on T cells that provide co-stimulatory signals, which are required for T cell activation. CD28 is the receptor for CD80 and CD86 . When activated by Toll-like receptor ligands, the CD80 expression is upregulated in antigen presenting cells...
receptor of the immune system
Immune system
An immune system is a system of biological structures and processes within an organism that protects against disease by identifying and killing pathogens and tumor cells. It detects a wide variety of agents, from viruses to parasitic worms, and needs to distinguish them from the organism's own...
's T cell
T cell
T cells or T lymphocytes belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B cells and natural killer cells , by the presence of a T cell receptor on the cell surface. They are...
s. CD28 is the co-receptor for the T cell receptor; It binds to receptors on the interacting partner in the reaction through one of its ligands (B7 family).
In its first human clinical trial
Clinical trial
Clinical trials are a set of procedures in medical research and drug development that are conducted to allow safety and efficacy data to be collected for health interventions...
s in March 2006, it caused catastrophic systemic organ failure in the subjects, despite being administered at a supposed sub-clinical dose of 0.1 mg per kg; some 500 times lower than the dose found safe in animals. Six volunteers were hospitalized on 13 March 2006, at least four of these suffering from multiple organ dysfunction
Multiple organ dysfunction syndrome
Multiple organ dysfunction syndrome ', previously known as multiple organ failure or multisystem organ failure , is altered organ function in an acutely ill patient requiring medical intervention to achieve homeostasis...
, and one trial volunteer is said to show signs of developing cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
.
The developing company, TeGenero Immuno Therapeutics
TeGenero
TeGenero AG was a pharmaceutical research company in Würzburg, Germany. It had 15 employees and was incorporated as an Aktiengesellschaft from 2002...
, entered into insolvency
Insolvency
Insolvency means the inability to pay one's debts as they fall due. Usually used to refer to a business, insolvency refers to the inability of a company to pay off its debts.Business insolvency is defined in two different ways:...
proceedings later in 2006. Tentative opinions from an as-yet uncompleted inquiry suggest that the problems resulted from "unforeseen biological action in humans", rather than breach of trial protocols, and the case therefore has had important ramifications for future trials of potentially powerful clinical agents.
Scientists in early 2007 put forth the theory that the drug acted in a different fashion in humans as compared with the laboratory animals in which the drug was first tried. The severe reactions in humans could have only occurred, they believe, in animals with memory T lymphocytes. Animals raised in a sterile lab would presumably have no 'memory' of previous illnesses, thus would not exhibit the severe reactions that occurred in the human subjects. However this is a misunderstanding of the research: the research says that non-human animals studied have fewer memory T cells than humans, and that stimulation through the CD28 receptor alone in memory T cells causes them to infiltrate organs and also activates them. In conjunction with the probable differences between the animal CD28 and the human CD28 protein sequence, and the fact that the antibody had been 'humanized' so as to be ignored by the human immune system which may not be similarly applicable in the experimental animal systems, one would expect differences in response. The surprise to the authorities and the firm was perhaps, ironically, that the drug was too powerful, and by starting with a dose regime based on animal studies rather than employing a more 'homeopathic' regime, they brought the industry into disrepute.
The drug, which was designated as an orphan medical product
Orphan drug
An orphan drug is a pharmaceutical agent that has been developed specifically to treat a rare medical condition, the condition itself being referred to as an orphan disease...
by the European Medicines Agency
European Medicines Agency
The European Medicines Agency is a European agency for the evaluation of medicinal products. From 1995 to 2004, the European Medicines Agency was known as European Agency for the Evaluation of Medicinal Products.Roughly parallel to the U.S...
in March 2005, was developed by TeGenero Immuno Therapeutics
TeGenero
TeGenero AG was a pharmaceutical research company in Würzburg, Germany. It had 15 employees and was incorporated as an Aktiengesellschaft from 2002...
, tested by Parexel
Parexel
PAREXEL International is a contract research organization , based in Lowell, Massachusetts and founded in 1982 by Josef H. von Rickenbach and Anne Sayigh. It provides services for companies in the pharmaceutical, biotechnology and medical device industries, including consulting, clinical studies...
and manufactured by Boehringer-Ingelheim
Boehringer-Ingelheim
C.H. Boehringer Sohn AG & Ko. KG is the parent company of Boehringer Ingelheim, which was founded in 1885 by Albert Boehringer in Ingelheim am Rhein. The Boehringer Ingelheim group is one of the world's 20 leading pharmaceutical companies. Headquartered in Ingelheim, Germany, it operates globally...
. TeGenero announced the first elucidation of the molecular structure of CD28 almost exactly one year prior to commencement of the TGN1412 phase I clinical trial.
Description of the drug
Mice of the inbred strain BALB/c were immunized with recombinant human CD28-Fc fusion proteins and boosted with a B lymphoma cell line transfected to express human CD28. Hybridomas were obtained by fusing B cells with the hybridoma partner X63Ag8.653 and screened for reactivity with human CD28 and TCR-independent mitogenic activity. Two monoclonals called 5.11A1 and 9D7 were identified. The more active of the two, 5.11A1, is a mouse IgG1 immunoglobulin. The complementarity determining regionComplementarity determining region
Complementarity determining regions are regions within antibodies or T cell receptors where these proteins complement an antigen's shape. Thus, CDRs determine the protein's affinity and specificity for specific antigens...
s of 5.11A1 were cloned into the framework of human IgG and combined with IgG1 (TGN1112) or IgG4 (TGN1412) constant regions. According to the company's Investigator Brochure, "TGN1412 is a humanised monoclonal antibody directed against the human CD28 antigen. The molecule was genetically engineered by transfer of the complementarity determining regions (CDRs) from heavy and light chain variable region sequences of a monoclonal mouse anti-humanC28 [sic] antibody (5.11A1, Luhder et al., 2003) into human heavy and light chain variable frameworks. Humanised variable regions were subsequently recombined with a human gene coding for the IgG4 gamma chain and with a human gene coding for a human kappa chain, respectively." The recombinant genes were transfected into Chinese hamster ovary cell
Chinese Hamster Ovary cell
Chinese hamster ovary cells are a cell line derived from the ovary of the Chinese hamster. They are often used in biological and medical research and commercially in the production of therapeutic proteins. They were introduced in the 1960s and grow as a cultured monolayer...
s and the recombinant antibody harvested from culture supernatant.
Swag
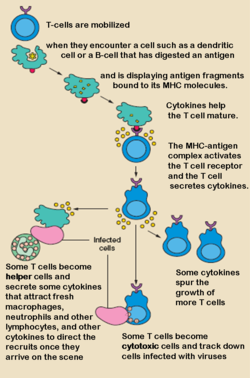
Mechanism of action

T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
(signal 1) and co-stimulation
Co-stimulation
During the activation of lymphocytes, co-stimulation is often crucial to the development of an effective immune response. Co-stimulation is required in addition to the antigen-specific signal from their antigen receptors.- Co-stimulation T cells require :...
(signal 2). Studies of monoclonal antibodies specific for mouse, rat, or human CD28 identified so-called "superagonistic" antibodies that could stimulate T cells without concurrent antigen-receptor stimulation (signal 1). Whether this activity represents a stronger activity or a different activity is uncertain. Two antibodies specific for human CD28 were identified. The more active of the two, TGN1112 (originally called 5.11A1), belonged to the IgG1 class of immunoglobulins. The other, TGN1412 (clone 9D7), belonged to the IgG4 class. The TCR-independent agonism of these antibodies involved binding to a specific part of the CD28 molecule called the C"D loop. It was initially hypothesized that an antibody with this property could be therapeutically useful in stimulating the immune system in immunosuppressed
Immunosuppression
Immunosuppression involves an act that reduces the activation or efficacy of the immune system. Some portions of the immune system itself have immuno-suppressive effects on other parts of the immune system, and immunosuppression may occur as an adverse reaction to treatment of other...
patients. However, in vitro
In vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
and in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
data from animal studies later suggested that administration would lead to preferential activation of regulatory T cell
Regulatory T cell
Regulatory T cells , sometimes known as suppressor T cells, are a specialized subpopulation of T cells which suppresses activation of the immune system and thereby maintains tolerance to self-antigens. The existence of regulatory T cells was the subject of significant controversy among...
s, leading to a net effect of T-cell downregulation. On its website, the company writes: "A pronounced T-cell activation and expansion mediated by CD28-SuperMAB in animal models is accompanied by the expression of anti-inflammatory cytokine
Cytokine
Cytokines are small cell-signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system and are a category of signaling molecules used extensively in intercellular communication...
s, like IL-10, rather than by the toxic cytokine storm
Cytokine storm
A cytokine storm, or hypercytokinemia is a potentially fatal immune reaction consisting of a positive feedback loop between cytokines and immune cells, with highly elevated levels of various cytokines.-Symptoms:...
of pro-inflammatory mediators induced by other agents that address the TCR complex.". As it turned out, the results of the first trial in humans indicate that this may not always be the case.
A new explanation for the trial mishap was suggested by the findings of a recent paper in Clinical Immunology. Pillai et al. found that all T cells that get activated using conventional TCR-mediated stimulation become regulatory for a brief time and express FOXP3. However, eventually most of these cells downregulate their regulatory capabilities and become effector cells. Thus, attempts to induce FOXP3+ T cells might also induce effector cells capable of causing tissue damage.
Other cells activated by CD28 ligation in humans are eosinophil granulocyte
Eosinophil granulocyte
Eosinophil granulocytes, usually called eosinophils or eosinophiles , are white blood cells that are one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. Along with mast cells, they also control mechanisms associated with...
s. They can release IFN-γ, IL-2, IL-4, and IL-13. However, most in vitro experiments are limited to the use of purified peripheral blood mononuclear cells (PBMN's) that do not contain those cells.
To function as an agonist, it has been suggested that TGN1412 needs to be a whole antibody
Antibody
An antibody, also known as an immunoglobulin, is a large Y-shaped protein used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. The antibody recognizes a unique part of the foreign target, termed an antigen...
, including the constant (Fc) region. According to a report by TeGenero, the F(ab)2 is not able to generate the required stimulation. Unlike the related clone TGN1112, an IgG1, TGN1412 is of the subclass IgG4. This choice was made as TGN1112 showed antibody-dependent cellular cytotoxicity on CD28+ Jurkat cells. Thus the function of antibody binding via an Fcγ receptor seems to be a requirement for the immune regulation. However, cell opsonisation by antibody leads normally to phagocytosis of the labeled cells, as seen in the case of HIV
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
.
Clinical trials
Phase I clinical trialClinical trial
Clinical trials are a set of procedures in medical research and drug development that are conducted to allow safety and efficacy data to be collected for health interventions...
s were conducted by Parexel at an independent clinical trials unit in leased space on the premises of Northwick Park and St. Mark's Hospital, London
London
London is the capital city of :England and the :United Kingdom, the largest metropolitan area in the United Kingdom, and the largest urban zone in the European Union by most measures. Located on the River Thames, London has been a major settlement for two millennia, its history going back to its...
, on 13 March 2006. Parexel is a company that carries out drug trials on behalf of pharmaceutical and biotechnology companies. Healthy volunteers were recruited to the study with a £2,000 fee, reportedly much higher than the 'few hundred quid' offered for other medical tests in the region. The trial resulted in hospitalization of all six volunteers administered the drug, at least four of whom suffered multiple organ dysfunction
Multiple organ dysfunction syndrome
Multiple organ dysfunction syndrome ', previously known as multiple organ failure or multisystem organ failure , is altered organ function in an acutely ill patient requiring medical intervention to achieve homeostasis...
.
The payment was seized upon by newspapers and reported in a sensationalist fashion. Good Clinical Practice (GCP) prohibits payments being made to Phase I trial volunteers on the basis of risk, and specifies that payments must be based upon the amount of time given up and the number of invasive procedures (e.g. blood sampling). For most trials, payments are in the range of around £1,000 per week - but the 'few hundred quid' trials mentioned above are those which the general public are most familiar with (since they only last a day or two, do not require working individuals to take time off work, and hence are more common).
The trial was a double-blind, randomized, placebo-controlled study
Placebo-controlled studies
A Placebo-controlled study is a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect...
, with two of the eight subjects receiving a placebo
Placebo
A placebo is a simulated or otherwise medically ineffectual treatment for a disease or other medical condition intended to deceive the recipient...
, and six receiving 1/500th of the highest dose used in previous experiments with cynomolgus macaques
Crab-eating Macaque
The Crab-eating macaque is a cercopithecine primate native to Southeast Asia. It is also called the "long-tailed macaque", and is referred to as the "cynomolgus monkey" in laboratories.-Etymology:...
. All six of the trial subjects who received the drug were male, aged 19 to 34 (median 29.5); none had a notable medical history, and all were well in the 2 weeks before the trial. The drug was given by intravenous infusion, starting at 8am, with an interval of around 10 minutes between patients, and each infusion lasting from 3 to 6 minutes. Roughly five minutes after the last participant had received his dose, the participant who had received the first dose complained of headache, and soon afterwards fever and pain. He took his shirt off, complaining that he felt like he was burning. Shortly after, the remaining participants who received the actual drug also became ill, vomiting and complaining of severe pain. The first patient was transferred to the Northwick Park hospital's intensive care unit 12 hours after infusion, with the others following within the next 4 hours. A severely affected volunteer, Mohammed Abdalla, a 28-year old who said he had hoped to set his brother up in business in Egypt, was described as having suffered a ballooned head. This led to his description as being similar to the "Elephant Man
Joseph Merrick
Joseph Carey Merrick , sometimes incorrectly referred to as John Merrick, was an English man with severe deformities who was exhibited as a human curiosity named the Elephant Man. He became well known in London society after he went to live at the London Hospital...
".
All of the men were reported to have experienced cytokine release syndrome
Cytokine release syndrome
Cytokine release syndrome is a common immediate complication occurring with the use of anti-T cell antibody infusions such as ATG, OKT3 and TGN1412. Severe cases are known as cytokine storms....
resulting in angioedema
Angioedema
Angioedema or Quincke's edema is the rapid swelling of the dermis, subcutaneous tissue, mucosa and submucosal tissues. It is very similar to urticaria, but urticaria, commonly known as hives, occurs in the upper dermis...
, swelling of skin
Skin
-Dermis:The dermis is the layer of skin beneath the epidermis that consists of connective tissue and cushions the body from stress and strain. The dermis is tightly connected to the epidermis by a basement membrane. It also harbors many Mechanoreceptors that provide the sense of touch and heat...
and mucous membrane
Mucous membrane
The mucous membranes are linings of mostly endodermal origin, covered in epithelium, which are involved in absorption and secretion. They line cavities that are exposed to the external environment and internal organs...
s, akin to the effects of the complement cascade in severe allergic reaction. The patients were treated with corticosteroid
Corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex. Corticosteroids are involved in a wide range of physiologic systems such as stress response, immune response and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte...
s to reduce inflammation, and plasma-exchange
Plasmapheresis
Plasmapheresis is the removal, treatment, and return of blood plasma from blood circulation. It is thus an extracorporeal therapy...
to attempt to remove TGN1412 from their circulation. The treating doctors confirmed in August 2006 that all six men had suffered from a cytokine storm
Cytokine storm
A cytokine storm, or hypercytokinemia is a potentially fatal immune reaction consisting of a positive feedback loop between cytokines and immune cells, with highly elevated levels of various cytokines.-Symptoms:...
, and that, paradoxically, the men's white blood cells had vanished almost completely several hours after administration of TGN1412.
According to a press release from 5 July 2006 on the North West London Hospitals NHS Trust website, where the men were treated, the patients continued to improve and "five of them went home within a month of the incident, while one patient remained in hospital until 26 June, when he also went home." However, Head of pharmacology at University College London Trevor Smart has suggested that the men may never fully recover, and may suffer long-term disruption to their immune systems.
An article by The Sunday Times
The Sunday Times (UK)
The Sunday Times is a Sunday broadsheet newspaper, distributed in the United Kingdom. The Sunday Times is published by Times Newspapers Ltd, a subsidiary of News International, which is in turn owned by News Corporation. Times Newspapers also owns The Times, but the two papers were founded...
on 30 July 2006 reported lawyers' claims that the long-term damage to the patients may be worse than originally thought. Medical assessment by immunologist Professor Richard Powell were said to have revealed that the blood of the patients contained a low number of regulatory T-cells, below one percent compared to three to five percent for healthy male adults - although the clinical significance of any such finding are unknown. Powell also reportedly claimed that one of the patients has "definite early signs that a lymphoid malignancy is developing". Some of the men involved in the trial are said to have been told that they face "a lifetime of contracting cancers and all the various auto-immune diseases from lupus
Lupus erythematosus
Lupus erythematosus is a category for a collection of diseases with similar underlying problems with immunity . Symptoms of these diseases can affect many different body systems, including joints, skin, kidneys, blood cells, heart, and lungs...
to MS
Multiple sclerosis
Multiple sclerosis is an inflammatory disease in which the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, leading to demyelination and scarring as well as a broad spectrum of signs and symptoms...
, from rheumatoid arthritis
Rheumatoid arthritis
Rheumatoid arthritis is a chronic, systemic inflammatory disorder that may affect many tissues and organs, but principally attacks synovial joints. The process produces an inflammatory response of the synovium secondary to hyperplasia of synovial cells, excess synovial fluid, and the development...
to ME
Chronic fatigue syndrome
Chronic fatigue syndrome is the most common name used to designate a significantly debilitating medical disorder or group of disorders generally defined by persistent fatigue accompanied by other specific symptoms for a minimum of six months, not due to ongoing exertion, not substantially...
."
TGN1412 had not previously been given to humans; however, the trial was preceded by animal testing, including in non-human primates
Primate
A primate is a mammal of the order Primates , which contains prosimians and simians. Primates arose from ancestors that lived in the trees of tropical forests; many primate characteristics represent adaptations to life in this challenging three-dimensional environment...
. The company claims that these did not indicate any safety issues. The US patent application states "it could be shown in a pilot study that an in vitro
In vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
administration of anti-human CD28-SuperMAB induces in a rhesus monkey in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
a profound activation of T cells, without clinically visible side effects" and goes on to say "This antibody—in spite of its strong T cell-stimulatory properties—is very well tolerated in vivo, in contrast to all other known T cell
T cell
T cells or T lymphocytes belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B cells and natural killer cells , by the presence of a T cell receptor on the cell surface. They are...
activating substances."
TeGenero
TeGenero
TeGenero AG was a pharmaceutical research company in Würzburg, Germany. It had 15 employees and was incorporated as an Aktiengesellschaft from 2002...
has apologized to the families involved, insists that these effects were completely unexpected, and said that all protocols have been followed. An investigation by the UK drug regulator reported that the reaction was not due to contamination of the dose, or an incorrect dose being administered, but suggested that the problem was due to "on target" effects of the drug. Criticism has been raised that six participants were given the drug in such a short time, which is against the recommendations of standard literature. However, the Medicines and Healthcare products Regulatory Agency
Medicines and Healthcare products Regulatory Agency
The Medicines and Healthcare products Regulatory Agency is the UK government agency which is responsible for ensuring that medicines and medical devices work and are acceptably safe....
(MHRA) has confirmed that they had approved the trial, including the protocol of giving the dose to all men within a short time. It appears the MHRA approved a protocol involving the doses being administrated between 8.00h-10.00h (i.e., 2 hours). One of the placebo-receiving participants has explained the doses were given with 2-minute intervals. Even though the participants were dosed with short intervals, this is not a deviation from the approved protocol.
The MHRA has further stated that the initial dose of TGN1412 was intended to be the first of a course of injections, with the dosage being ramped up over time. It has been reported that the initial dose was one five-hundredth of that which the animal studies indicated was a maximum safe dose. Dr. David Glover, an industry consultant, has suggested that because the antibody was raised against human CD28, the safe dosage may have been lower in humans than in animals.
Criticism and controversy
Not much information has been released to the public about the ongoing investigations. At the moment, there appear to be two issues. There is the issue of the trial protocol of giving the drug to six participants within a short time. While this was approved by the MHRA, as mentioned earlier, it seems a two hour protocol was approved, but that the drug was administered to all participants within just twenty minutes, based on the statement of a study participant. It appears that neither the companies involved nor the authorities have commented on this point yet.Another issue is whether the company should have known the drug would provoke this reaction in humans. The comments on the company webpage and in the patent application at least indicate the company knew this type of drug could cause a cytokine storm
Cytokine storm
A cytokine storm, or hypercytokinemia is a potentially fatal immune reaction consisting of a positive feedback loop between cytokines and immune cells, with highly elevated levels of various cytokines.-Symptoms:...
. An immunologist contacted by New Scientist
New Scientist
New Scientist is a weekly non-peer-reviewed English-language international science magazine, which since 1996 has also run a website, covering recent developments in science and technology for a general audience. Founded in 1956, it is published by Reed Business Information Ltd, a subsidiary of...
and who wished to be anonymous has commented that “You don’t need to be a rocket scientist to work out what will happen if you non-specifically activate every T cell in the body.”
While the drug had appeared to be safe in animal models, researchers have noted that there are reasons why these may not be indicative of the response in humans, particularly with respect to this type of drug. The BBC reported that “two of 20 monkeys used in earlier tests suffered an increase in the size of lymph node
Lymph node
A lymph node is a small ball or an oval-shaped organ of the immune system, distributed widely throughout the body including the armpit and stomach/gut and linked by lymphatic vessels. Lymph nodes are garrisons of B, T, and other immune cells. Lymph nodes are found all through the body, and act as...
s,” but that “this information was given to the men and submitted to the test regulators.” TeGenero say this was transient and was evidence of the extra T cells that the drug produces. Experiments with another drug affecting the CD28 receptor (but to a lesser extent than TGN1412) had also shown side effects in human trials. There have been criticisms that the risks taken and the design of the protocol were insufficiently justified by proper statistical evidence
Statistics
Statistics is the study of the collection, organization, analysis, and interpretation of data. It deals with all aspects of this, including the planning of data collection in terms of the design of surveys and experiments....
.
The MHRA's views
On 5 April 2006, the Medicines and Healthcare Products Regulatory Agency issued an interim report on the TGN1412 trial. They found no deficiencies in TeGenero’s pre-clinical work; there was no evidence of undisclosed studies. Parexel’s records and processes appeared in order (including dose measurement and administration) and the MHRA felt that their actions did not contribute to the serious adverse events, nor were there any deficiencies in the animal work; results accurately reflected the raw data.German regulatory authorities inspected the production of the material by Boehringer Ingelheim, looking at the manufacture, testing, storage and distribution of the TGN1412. No deficiencies were identified which could have contributed to the serious adverse effects. Although tests are ongoing on the actual material used, the MHRA state that tests are consistent with the TGN1412 being up to specification at the moment.
The MHRA have concluded that the most likely cause of the reaction in trial subjects was an unpredicted biological action of the drug in humans. The interim report recognises that important scientific and medical questions about the risks of testing these agents in human subjects have been raised. To that end, the UK Secretary of State for Health has agreed to establish a group of leading international experts to consider those issues and to provide a report on the future authorisation of such trials (with an interim report at three months).
Until the expert group has reported, all further clinical trial applications involving first-in-humans trials of any monoclonal antibody or other novel molecules targeting the immune system will not be authorised in the UK, without having had additional expert opinion on whether the effects seen in the TGN1412 may be repeated. There will be a fuller report on the TGN1412 trial in future, but the expert group will run concurrently.
See also
- Adverse effect (medicine)Adverse effect (medicine)In medicine, an adverse effect is a harmful and undesired effect resulting from a medication or other intervention such as surgery.An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. If it results from an unsuitable or incorrect dosage or...
- CD28CD28CD28 is one of the molecules expressed on T cells that provide co-stimulatory signals, which are required for T cell activation. CD28 is the receptor for CD80 and CD86 . When activated by Toll-like receptor ligands, the CD80 expression is upregulated in antigen presenting cells...
- Clinical trial protocolClinical trial protocolA clinical trial protocol is a document that describes the objective, design, methodology, statistical considerations, and organization of a clinical trial...
- PharmacovigilancePharmacovigilancePharmacovigilance is the pharmacological science relating to the detection, assessment, understanding and prevention of adverse effects, particularly long term and short term side effects of medicines...
- First-in-man studyFirst-in-man studyA first-in-man study is a clinical trial where a medical procedure, previously developed and assessed through in vitro or animal testing, or through mathematical modelling is tested on human subjects for the first time....
- Co-stimulationCo-stimulationDuring the activation of lymphocytes, co-stimulation is often crucial to the development of an effective immune response. Co-stimulation is required in addition to the antigen-specific signal from their antigen receptors.- Co-stimulation T cells require :...
- Drug developmentDrug developmentDrug development is a blanket term used to define the process of bringing a new drug to the market once a lead compound has been identified through the process of drug discovery...
- Pre-clinical developmentPre-clinical developmentIn drug development, pre-clinical development is a stage of research that begins before clinical trials can begin, and during which important feasibility, iterative testing and drug safety data is collected....
- EudraVigilanceEudraVigilanceEudraVigilance is the European data processing network and management system for reporting and evaluation of suspected adverse reactions during the development of new drugs and for following the marketing authorisation of medicinal products in the European Economic Area .The European...
External links
- Patent applications for TGN1412
- Report in Nature on TGN1412
- BBC News: Drug trial man 'may lose fingers'
- BBC News: Regulators slam drug trial firm
- Channel 4: The Drug Trial That Went Wrong
- Nature news: Animal tests may have missed danger because monkeys 'too clean'
- crisis communications case study of Tegenero clinical trial
- Further lessons from the TGN1412 tragedy

