
Synthetic molecular motors
Encyclopedia
Synthetic molecular motors are molecular machine
s capable of rotation under energy input. Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion (via protein dynamics), some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors. The prospect of synthetic molecular motors was first raised by the nanotechnology
pioneer Richard Feynman
in 1959 in his talk There's Plenty of Room at the Bottom
.
The basic requirements for a synthetic motor are repetitive 360° motion, the consumption of energy and unidirectional rotation. The first two efforts in this direction, the chemically driven motor by Dr. T. Ross Kelly of Boston College with co-workers and the light-driven motor by Feringa and co-workers, were published in 1999 in the same issue of Nature
. In 2008 Petr Král and co-workers proposed electron tunneling motors continuously rotated by a permanent torque
, opening the possibility of practical realization of a real molecular motor machine. It is expected that reports of more efforts in this field will increase as understanding of chemistry and physics at the nanolevel improves.
rotor and a helicene
, and is capable of performing a unidirectional 120° rotation.
This rotation takes place in five steps. The amine
group present on the triptycene moiety is converted to an isocyanate
group by condensation with phosgene
(a). Thermal or spontaneous rotation around the central bond then brings the isocyanate group in proximity of the hydroxyl
group located on the helicene moiety (b), thereby allowing these two groups to react with each other (c). This reaction irreversibly
traps the system as a strained
cyclic urethane
that is higher in energy and thus energetically closer to the rotational energy barrier than the original state. Further rotation of the triptycene moiety therefore requires only a relatively small amount of thermal activation
in order to overcome this barrier, thereby releasing the strain (d). Finally, cleavage of the urethane group restores the amine and alcohol functionalities
of the molecule (e).
The result of this sequence of events is a unidirectional 120° rotation of the triptycene moiety with respect to the helicene moiety. Additional forward or backward rotation of the triptycene rotor is inhibited by the helicene moiety, which serves a function similar to that of the pawl of a ratchet
. The unidirectionality of the system is a result from both the asymmetric skew of the helicene moiety as well as the strain of the cyclic urethane which is formed in c. This strain can be only be lowered by the clockwise rotation of the triptycene rotor in d, as both counterclockwise rotation as well as the inverse process of d are energetically unfavorable. In this respect the preference for the rotation direction is determined by both the positions of the functional groups and the shape of the helicene and is thus built into the design of the molecule instead of dictated by external factors.
The motor by Kelly and co-workers is an elegant example of how chemical energy
can be used to induce controlled, unidirectional rotational motion, a process which resembles the consumption of ATP
in organisms in order to fuel numerous processes. However, it does suffer from a serious drawback: the sequence of events that leads to 120° rotation is not repeatable. Kelly and co-workers have therefore searched for ways to extend the system so that this sequence can be carried out repeatedly. Unfortunately, their attempts to accomplish this objective have not been successful and currently the project has been abandoned.
Two additional examples of synthetic chemically driven rotary molecular motors that have been reported in literature make use of the stereoselective
ring opening of a racemic biaryl lactone
by the use of chiral reagents, which results in a directed 90° rotation of one aryl with respect to the other aryl. Branchaud and co-workers have reported that this approach, followed by an additional ring closing step, can be used to accomplish a non-repeatable 180° rotation. After watching Branchaud's talk on this approach to molecular motors, at a 2005 ACS meeting, Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation. The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the aryl
moiety is restricted, although helix
inversion is possible. In stages B and D the aryl can rotate with respect to the naphthalene
with steric interactions
preventing the aryl from passing the naphthalene. The rotary cycle consists of four chemically induced steps which realize the conversion of one stage into the next. Steps 1 and 3 are asymmetric ring opening reactions which make use of a chiral reagent in order to control the direction of the rotation of the aryl. Steps 2 and 4 consist of the deprotection
of the phenol
, followed by regioselective
ring formation. So far this molecular motor is the only reported example of a fully chemically driven artificial rotary molecular motor that is capable of 360° rotation.
, The Netherlands
reported the creation of a unidirectional molecular rotor. Their 360° molecular motor system consists of a bis-helicene
connected by an alkene
double bond displaying axial chirality
and having two stereocenter
s.
One cycle of unidirectional rotation takes 4 reaction steps. The first step is a low temperature endothermic photoisomerization of the trans (P,P) isomer 1 to the cis (M,M) 2 where P stands for the right-handed helix
and M for the left-handed helix. In this process, the two axial methyl groups are converted into two less sterically favorable equatorial methyl groups.
By increasing the temperature to 20 °C these methyl groups convert back exothermally
to the (P,P) cis axial groups (3) in a helix inversion. Because the axial isomer is more stable than the equatorial isomer, reverse rotation is blocked. A second photoisomerization converts (P,P) cis 3 into (M,M) trans 4, again with accompanying formation of sterically unfavorable equatorial methyl groups. A thermal isomerization process at 60 °C closes the 360° cycle back to the axial positions.
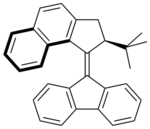 A major hurdle to overcome is the long reaction time for complete rotation in these systems, which does not compare to rotation speeds displayed by motor proteins in biological systems. In the fastest system to date, with a fluorene
A major hurdle to overcome is the long reaction time for complete rotation in these systems, which does not compare to rotation speeds displayed by motor proteins in biological systems. In the fastest system to date, with a fluorene
lower half, the half-life of the thermal helix inversion is 0.005 seconds. This compound is synthesized using the Barton-Kellogg reaction
. In this molecule the slowest step in its rotation, the thermally induced helix-inversion, is believed to proceed much more quickly because the larger tert-butyl group makes the unstable isomer even less stable than when the methyl group is used. This is because the unstable isomer is more destabilized than the transition state that leads to helix-inversion. The different behaviour of the two molecules is illustrated by the fact that the half-life time for the compound with a methyl group instead of a tert-butyl group is 3.2 minutes.
The Feringa principle has been incorporated into a prototype nanocar
. The car synthesized
has a helicene-derived engine with an oligo (phenylene ethynylene) chassis and four carborane
wheels and is expected to be able to move on a solid surface with scanning tunneling microscopy monitoring, although so far this has not been observed. The motor does not perform with fullerene
wheels because they quench the photochemistry of the motor moiety. The ability of certain Feringa systems to act as an asymmetric catalyst has also been demonstrated.
is an electrically operated motor which is made from a single butyl
methyl sulphide
molecule. The molecule is adsorbed onto a Copper
(111) single crystal
piece by chemisorption
. The motor, the world's smallest electric motor, is just a nanometer (billionth of a meter) across (60,000 times smaller than the thickness of a human hair). It was developed by chemists at the Tufts University School of Arts and Sciences
.
Molecular machine
A molecular machine, or nanomachine, is any discrete number of molecular components that produce quasi-mechanical movements in response to specific stimuli . The expression is often more generally applied to molecules that simply mimic functions that occur at the macroscopic level...
s capable of rotation under energy input. Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion (via protein dynamics), some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors. The prospect of synthetic molecular motors was first raised by the nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
pioneer Richard Feynman
Richard Feynman
Richard Phillips Feynman was an American physicist known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics and the physics of the superfluidity of supercooled liquid helium, as well as in particle physics...
in 1959 in his talk There's Plenty of Room at the Bottom
There's Plenty of Room at the Bottom
There's Plenty of Room at the Bottom is the title of a lecture given by physicist Richard Feynman at an American Physical Society meeting at Caltech on December 29, 1959...
.
The basic requirements for a synthetic motor are repetitive 360° motion, the consumption of energy and unidirectional rotation. The first two efforts in this direction, the chemically driven motor by Dr. T. Ross Kelly of Boston College with co-workers and the light-driven motor by Feringa and co-workers, were published in 1999 in the same issue of Nature
Nature (journal)
Nature, first published on 4 November 1869, is ranked the world's most cited interdisciplinary scientific journal by the Science Edition of the 2010 Journal Citation Reports...
. In 2008 Petr Král and co-workers proposed electron tunneling motors continuously rotated by a permanent torque
Torque
Torque, moment or moment of force , is the tendency of a force to rotate an object about an axis, fulcrum, or pivot. Just as a force is a push or a pull, a torque can be thought of as a twist....
, opening the possibility of practical realization of a real molecular motor machine. It is expected that reports of more efforts in this field will increase as understanding of chemistry and physics at the nanolevel improves.
Chemically driven rotary molecular motors
A first example of a synthetic chemically driven rotary molecular motor was reported by Kelly and co-workers in 1999. Their system is made up from a three-bladed triptyceneTriptycene
Triptycenes are a class of aromatic hydrocarbons. The parent compound triptycene is the Diels-Alder reaction product of anthracene and benzyne. The compound has a paddlewheel configuration with D3h symmetry...
rotor and a helicene
Helicene
Helicenes in organic chemistry are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped molecules...
, and is capable of performing a unidirectional 120° rotation.
This rotation takes place in five steps. The amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group present on the triptycene moiety is converted to an isocyanate
Isocyanate
Isocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
group by condensation with phosgene
Phosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
(a). Thermal or spontaneous rotation around the central bond then brings the isocyanate group in proximity of the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group located on the helicene moiety (b), thereby allowing these two groups to react with each other (c). This reaction irreversibly
Irreversibility
In science, a process that is not reversible is called irreversible. This concept arises most frequently in thermodynamics, as applied to processes....
traps the system as a strained
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional...
cyclic urethane
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
that is higher in energy and thus energetically closer to the rotational energy barrier than the original state. Further rotation of the triptycene moiety therefore requires only a relatively small amount of thermal activation
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
in order to overcome this barrier, thereby releasing the strain (d). Finally, cleavage of the urethane group restores the amine and alcohol functionalities
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
of the molecule (e).
The result of this sequence of events is a unidirectional 120° rotation of the triptycene moiety with respect to the helicene moiety. Additional forward or backward rotation of the triptycene rotor is inhibited by the helicene moiety, which serves a function similar to that of the pawl of a ratchet
Ratchet (device)
A ratchet is a device that allows continuous linear or rotary motion in only one direction while preventing motion in the opposite direction. Because most socket wrenches today use ratcheting handles, the term "ratchet" alone is often used to refer to a ratcheting wrench, and the terms "ratchet"...
. The unidirectionality of the system is a result from both the asymmetric skew of the helicene moiety as well as the strain of the cyclic urethane which is formed in c. This strain can be only be lowered by the clockwise rotation of the triptycene rotor in d, as both counterclockwise rotation as well as the inverse process of d are energetically unfavorable. In this respect the preference for the rotation direction is determined by both the positions of the functional groups and the shape of the helicene and is thus built into the design of the molecule instead of dictated by external factors.
The motor by Kelly and co-workers is an elegant example of how chemical energy
Chemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
can be used to induce controlled, unidirectional rotational motion, a process which resembles the consumption of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
in organisms in order to fuel numerous processes. However, it does suffer from a serious drawback: the sequence of events that leads to 120° rotation is not repeatable. Kelly and co-workers have therefore searched for ways to extend the system so that this sequence can be carried out repeatedly. Unfortunately, their attempts to accomplish this objective have not been successful and currently the project has been abandoned.
Two additional examples of synthetic chemically driven rotary molecular motors that have been reported in literature make use of the stereoselective
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during the non-stereospecific creation of a new stereocenter or during the non-stereospecific transformation of a pre-existing one...
ring opening of a racemic biaryl lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
by the use of chiral reagents, which results in a directed 90° rotation of one aryl with respect to the other aryl. Branchaud and co-workers have reported that this approach, followed by an additional ring closing step, can be used to accomplish a non-repeatable 180° rotation. After watching Branchaud's talk on this approach to molecular motors, at a 2005 ACS meeting, Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation. The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
moiety is restricted, although helix
Helicity
The term helicity has several meanings. In physics, all referring to a phenomenon that resembles a helix. See:*helicity , the extent to which corkscrew-like motion occurs...
inversion is possible. In stages B and D the aryl can rotate with respect to the naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
with steric interactions
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
preventing the aryl from passing the naphthalene. The rotary cycle consists of four chemically induced steps which realize the conversion of one stage into the next. Steps 1 and 3 are asymmetric ring opening reactions which make use of a chiral reagent in order to control the direction of the rotation of the aryl. Steps 2 and 4 consist of the deprotection
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
of the phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
, followed by regioselective
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
ring formation. So far this molecular motor is the only reported example of a fully chemically driven artificial rotary molecular motor that is capable of 360° rotation.
Light-driven rotary molecular motors
In 1999 the laboratory of Prof. Dr. Ben L. Feringa at the University of GroningenUniversity of Groningen
The University of Groningen , located in the city of Groningen, was founded in 1614. It is one of the oldest universities in the Netherlands as well as one of its largest. Since its inception more than 100,000 students have graduated...
, The Netherlands
Netherlands
The Netherlands is a constituent country of the Kingdom of the Netherlands, located mainly in North-West Europe and with several islands in the Caribbean. Mainland Netherlands borders the North Sea to the north and west, Belgium to the south, and Germany to the east, and shares maritime borders...
reported the creation of a unidirectional molecular rotor. Their 360° molecular motor system consists of a bis-helicene
Helicene
Helicenes in organic chemistry are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped molecules...
connected by an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
double bond displaying axial chirality
Axial chirality
Axial chirality is a special case of chirality in which a molecule does not possess a stereogenic center but an axis of chirality – an axis about which a set of substituents is held in a spatial arrangement that is not superposable on its mirror image...
and having two stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s.
One cycle of unidirectional rotation takes 4 reaction steps. The first step is a low temperature endothermic photoisomerization of the trans (P,P) isomer 1 to the cis (M,M) 2 where P stands for the right-handed helix
Helix
A helix is a type of smooth space curve, i.e. a curve in three-dimensional space. It has the property that the tangent line at any point makes a constant angle with a fixed line called the axis. Examples of helixes are coil springs and the handrails of spiral staircases. A "filled-in" helix – for...
and M for the left-handed helix. In this process, the two axial methyl groups are converted into two less sterically favorable equatorial methyl groups.
By increasing the temperature to 20 °C these methyl groups convert back exothermally
Exothermic reaction
An exothermic reaction is a chemical reaction that releases energy in the form of light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation:-Overview:...
to the (P,P) cis axial groups (3) in a helix inversion. Because the axial isomer is more stable than the equatorial isomer, reverse rotation is blocked. A second photoisomerization converts (P,P) cis 3 into (M,M) trans 4, again with accompanying formation of sterically unfavorable equatorial methyl groups. A thermal isomerization process at 60 °C closes the 360° cycle back to the axial positions.

Fluorene
Fluorene, or 9H-fluorene, is a polycyclic aromatic hydrocarbon. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It is combustible. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar...
lower half, the half-life of the thermal helix inversion is 0.005 seconds. This compound is synthesized using the Barton-Kellogg reaction
Barton-Kellogg reaction
The Barton-Kellogg reaction is a coupling reaction between a ketone and a thioketone through a diazo intermediate forming an alkene.This reaction has been pioneered by Hermann Staudinger and therefore the reaction also goes by the name Staudinger type diazo-thioketone coupling.- Reaction mechanism...
. In this molecule the slowest step in its rotation, the thermally induced helix-inversion, is believed to proceed much more quickly because the larger tert-butyl group makes the unstable isomer even less stable than when the methyl group is used. This is because the unstable isomer is more destabilized than the transition state that leads to helix-inversion. The different behaviour of the two molecules is illustrated by the fact that the half-life time for the compound with a methyl group instead of a tert-butyl group is 3.2 minutes.
The Feringa principle has been incorporated into a prototype nanocar
Nanocar
The nanocar is a molecule designed in 2005 at Rice University by a group headed by Professor James Tour. Despite the name, the original nanocar does not contain a molecular motor, hence, it is not really a car...
. The car synthesized
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
has a helicene-derived engine with an oligo (phenylene ethynylene) chassis and four carborane
Carborane
A carborane is a cluster composed of boron and carbon atoms. Like many of the related boranes, these clusters are polyhedra and are similarly classified as closo-, nido-, arachno-, hypho-, etc...
wheels and is expected to be able to move on a solid surface with scanning tunneling microscopy monitoring, although so far this has not been observed. The motor does not perform with fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
wheels because they quench the photochemistry of the motor moiety. The ability of certain Feringa systems to act as an asymmetric catalyst has also been demonstrated.
Experimental demonstration of a single molecule-electric motor
The single molecule electric motorSingle molecule electric motor
The single molecule electric motor is an electrically operated motor which is made from a single butyl methyl sulphide molecule. The molecule is adsorbed onto a Copper single crystal piece by chemisorption. The motor, the world's smallest electric motor, is just a nanometer across...
is an electrically operated motor which is made from a single butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
methyl sulphide
Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
molecule. The molecule is adsorbed onto a Copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
(111) single crystal
Single crystal
A single crystal or monocrystalline solid is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries...
piece by chemisorption
Chemisorption
Chemisorption is a sub-class of adsorption, driven by a chemical reaction occurring at the exposed surface. A new chemical species is generated at the adsorbant surface...
. The motor, the world's smallest electric motor, is just a nanometer (billionth of a meter) across (60,000 times smaller than the thickness of a human hair). It was developed by chemists at the Tufts University School of Arts and Sciences
Tufts University School of Arts and Sciences
The School of Arts and Sciences is the largest of the eight schools and colleges that comprise Tufts University. Together with the School of Engineering, it offers undergraduate and graduate degrees in the liberal arts, sciences, and engineering...
.

