
Helicene
Encyclopedia

Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
are ortho-condensed polycyclic
Polycyclic compound
In organic chemistry, a polycyclic compound is a cyclic compound with more than one hydrocarbon loop or ring structures . In general, the term includes all polycyclic aromatic compounds, including the polycyclic aromatic hydrocarbons, the heterocyclic aromatic compounds containing sulfur,...
aromatic compounds
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
in which benzene rings
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
or other aromatics are angularly annulated
Annulation
Annulation in organic chemistry is a chemical reaction in which a new ring is constructed on another molecule ....
to give helically
Helix
A helix is a type of smooth space curve, i.e. a curve in three-dimensional space. It has the property that the tangent line at any point makes a constant angle with a fixed line called the axis. Examples of helixes are coil springs and the handrails of spiral staircases. A "filled-in" helix – for...
-shaped molecules. The chemistry of helicenes has attracted continuing attention because of their unique structural, spectral
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, and optical
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
features.
Helicenes are notable for having chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
while lacking both asymmetric carbons
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
and chiral centers. Helicenes' chirality results from the fact that clockwise and counterclockwise helices are non-superimposable
Superimposition
In graphics, superimposition is the placement of an image or video on top of an already-existing image or video, usually to add to the overall image effect, but also sometimes to conceal something .This technique is used in cartography to produce photomaps by superimposing grid lines, contour lines...
– this is an example of axial chirality
Axial chirality
Axial chirality is a special case of chirality in which a molecule does not possess a stereogenic center but an axis of chirality – an axis about which a set of substituents is held in a spatial arrangement that is not superposable on its mirror image...
.
Background
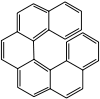
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
by M. S. Newman
Melvin Spencer Newman
Melvin Spencer Newman was an American chemist, best known for inventing the Newman projection. He was born in New York City, but shortly after his family moved to New Orleans, Louisiana. When he was 14, they moved back to New York, where he attended Riverdale County School. From 1925 to 1932 he...
and D. Lednicer in 1956 via a scheme that closed the two central rings by Friedel-Crafts cyclization
Friedel-Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877. There are two main types of Friedel–Crafts reactions: alkylation reactions and acylation reactions. This reaction type is a form of electrophilic aromatic substitution...
of carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
compounds. Since then, several methods for synthesizing helicenes have been reported. Today, the synthesis of helicenes with different lengths and substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s is possible. The oxidative photocyclization
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
of a stilbene
Stilbene
-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
-type precursor
Precursor (chemistry)
In chemistry, a precursor is a compound that participates in the chemical reaction that produces another compound. In biochemistry, the term "precursor" is used more specifically to refer to a chemical compound preceding another in a metabolic pathway....
is used most often as the key step. The longest helicene, [14]helicene, was prepared in 1975 by this method.
In one study, [5]helicene was synthesized in an olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
reaction of a divinyl compound (prepared from 1,1'-bi-2-naphthol
1,1'-Bi-2-naphthol
1,1'-Bi-2-naphthol is an organic compound that is often used as a ligand for transition-metal catalysed asymmetric synthesis. BINOL has axial chirality and the two enantiomers can be readily separated and are stable toward racemisation. The specific rotation of the two enantiomers is +/- 35.5°...
(BINOL) in several steps), with Grubbs' second generation catalyst
Grubbs' catalyst
Grubbs' Catalyst is a transition metal carbene complex named after Robert H. Grubbs, the chemist who first synthesized it. There are two generations of the catalyst, as shown on the right. In contrast to other olefin metathesis catalysts, Grubbs' Catalysts tolerate other functional groups in the...
:

Analog (chemistry)
In chemistry, a structural analog , also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced...
s of helicenes.
Circulenes
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
s are called circulenes. [5]circulene or corannulene
Corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of...
, [6]circulene or coronene
Coronene
Coronene is a polycyclic aromatic hydrocarbon comprising six peri-fused benzene rings. Its chemical formula is . It is a yellow material that dissolves in such solvents as benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light...
, [7]circulene and [12]circulene (kekulene
Kekulene
Kekulene is a polycyclic aromatic hydrocarbon with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule....
) have been synthesized in the laboratory. These compounds belong to a larger class of geodesic polyarene
Geodesic polyarene
A geodesic polyarene in organic chemistry is a polycyclic aromatic hydrocarbon with curved convex or concave surfaces. Examples are fullerenes, nanotubes, corannulenes, helicenes and sumanene...
s. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle
Saddle
A saddle is a supportive structure for a rider or other load, fastened to an animal's back by a girth. The most common type is the equestrian saddle designed for a horse, but specialized saddles have been created for camels and other creatures...
-shaped structure (compare to cones and partial cones in calixarene
Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde. The word calixarene is derived from calix or chalice because this type of molecule resembles a vase and from the word arene that refers to the aromatic building block...
s).
Sulflower
A stable octacirculene based on thiopheneThiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
, not containing any hydrogen, has been reported with the name sulflower (a portmanteau of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and sunflower
Sunflower
Sunflower is an annual plant native to the Americas. It possesses a large inflorescence . The sunflower got its name from its huge, fiery blooms, whose shape and image is often used to depict the sun. The sunflower has a rough, hairy stem, broad, coarsely toothed, rough leaves and circular heads...
). With molecular formula (C2S)8 the compound is considered an analogue of carbon sulfide. The molecule is flat and together with the 9-membered homologue is at a local strain energy
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
minimum.
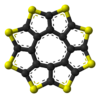 |
 |
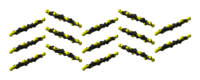 |
 |
Ball-and-stick model In chemistry, the ball-and-stick model is a molecular model of a chemical substance which is to display both the three-dimensional position of the atoms and the bonds between them... of the sulflower molecule |
Space-filling model In chemistry a space-filling model, also known as calotte model, is a type of three-dimensional molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic... of the sulflower molecule |
molecules in the crystal structure |
molecules in the crystal structure |
Its synthesis (a variation of the Ferrario reaction) is based on deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of a tetrathiophene with lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
followed by reaction with elemental sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
to a sulfur-substituted intermediate followed by vacuum pyrolysis.
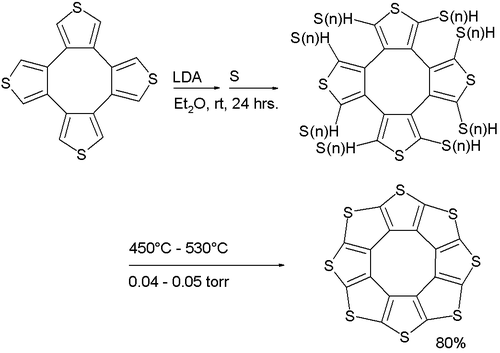
Sulflower molecules have a perfect planar structure with high symmetry (D8h). Because of their planar structure, they are predicted to store a lot of hydrogen molecules between the stacks. The conformation of the H2 molecule is calculated to be "standing up" over the five membered rings. Detailed DFT calculations have been performed on these molecules.

