
Single-molecule magnet
Encyclopedia
Single-molecule magnets or SMMs are a class of metalorganic compounds
, that show superparamagnetic
behavior below a certain blocking temperature at the molecular scale. In this temperature range, SMMs exhibit magnetic hysteresis of purely molecular origin . Contrary to conventional bulk magnets and molecule-based magnets, collective long-range magnetic ordering of magnetic moment
s is not necessary .
interactions and can be described by the following isotropic Heisenberg Hamiltonian
:

where is the coupling constant between spin i (operator
is the coupling constant between spin i (operator  ) and spin j (operator
) and spin j (operator  ). For positive J the coupling is called ferromagnetic (parallel alignment of spins) and for negative J the coupling is called antiferromagnetic (antiparallel alignment of spins).
). For positive J the coupling is called ferromagnetic (parallel alignment of spins) and for negative J the coupling is called antiferromagnetic (antiparallel alignment of spins).
The combination of these properties can lead to an energy barrier so that, at low temperatures, the system can be trapped in one of the high-spin energy wells.
"These molecules contain a finite number of interacting spin centers (e.g. paramagnetic ions) and thus provide ideal opportunities to study basic concepts of magnetism
. Some of them possess magnetic ground states and give rise to hysteresis
effects and metastable magnetic phases. They may show quantum tunneling of the magnetization which raises the question of coherent dynamics in such systems. Other types of molecules exhibit pronounced frustration effects, whereas so-called spin crossover
substances can switch their magnetic ground state and related properties such as color under irradiation of laser light, pressure or heat. Scientists from various fields – chemistry, physics; theory and experiment – have joined the research on molecular magnetism in order to explore the unprecedented properties of these new compounds."
"Single-molecule magnets (SMMs) have many important advantages over conventional nanoscale magnetic particles composed of metal
s, metal alloys or metal oxides. These advantages include uniform size, solubility in organic solvents, and readily alterable peripheral ligand
s, among others."
"A single molecule magnet is an example of a macroscopic quantum system. [...] If we could detect spin flips in a single atom or molecule, we could use the spin to store information. This would enable us to increase the storage capacity of computer hard disk
s. [...] A good starting point for trying to detect spin flips is to find a molecule with a spin of several Bohr magnetons. [An electron has an intrinsic magnetic dipole moment of approximately one Bohr magneton.] There is a very well studied molecular magnet, Mn12-acetate, which has a spin S = 10 (Figure 3). This molecule is a disc-shaped organic molecule in which twelve Mn ions are embedded. Eight of these form a ring, each having a charge of +3 and a spin S = 2. The other four form a tetrahedron
, each having a charge of +4 and a spin S = 3/2. The exchange interactions within the molecule are such that the spins of the ring align themselves in opposition to the spins of the tetrahedron, giving the molecule a total net spin S = 10."
"The ability of a single molecule to behave like a tiny magnet (single molecular magnets, SMMs) has seen a rapid growth in research over the last few years. SMMs represent the smallest possible magnetic devices and are a controllable, bottom-up approach to nanoscale magnetism. Potential applications of SMMs include quantum computing
, high-density information storage and magnetic refrigeration
."
 "A single molecule magnet is an example of a macroscopic quantum system. [...] If we could detect spin flips in a single atom or molecule, we could use the spin to store information. This would enable us to increase the storage capacity of computer hard disk
"A single molecule magnet is an example of a macroscopic quantum system. [...] If we could detect spin flips in a single atom or molecule, we could use the spin to store information. This would enable us to increase the storage capacity of computer hard disk
s. [...] A good starting point for trying to detect spin flips is to find a molecule with a spin of several Bohr magnetons. [An electron has an intrinsic magnetic dipole moment of approximately one Bohr magneton.] There is a very well studied molecular magnet, Mn12-acetate, which has a spin S = 10 (Figure 3). This molecule is a disc-shaped organic molecule in which twelve Mn ions are embedded. Eight of these form a ring, each having a charge of +3 and a spin S = 2. The other four form a tetrahedron
, each having a charge of +4 and a spin S = 3/2. The exchange interactions within the molecule are such that the spins of the ring align themselves in opposition to the spins of the tetrahedron, giving the molecule a total net spin S = 10."
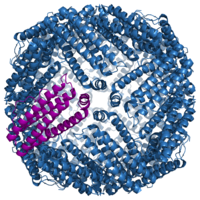 The archetype of single-molecule magnets is called "Mn12". It is a polymetallic manganese
The archetype of single-molecule magnets is called "Mn12". It is a polymetallic manganese
(Mn) complex having the formula [Mn12O12(OAc)16(H2O)4], where OAc stands for acetate
. It has the remarkable property of showing an extremely slow relaxation of their magnetization below a blocking temperature. [Mn12O12(OAc)16(H2O)4]·4H2O·2AcOH which is called "Mn12-acetate" is a common form of this used in research.
"Mn4" is another researched type single-molecule magnet. Three of these are:
In each of these Mn4 complexes "there is a planar diamond core of MnIII2MnII2 ions. An analysis of the variable-temperature and variable-field magnetization data indicate that all three molecules have intramolecular ferromagnetic coupling and a S = 9 ground state. The presence of a frequency-dependent alternating current susceptibility signal indicates a significant energy barrier between the spin-up and spin-down states for each of these three MnIII2MnII2 complexes."
Single-molecule magnets are also based on iron
clusters because they potentially have large spin states. In addition the biomolecule
ferritin
is also considered a nanomagnet
. In the cluster Fe8Br the cation Fe8 stands for [Fe8O2(OH)12(tacn)6]8+ with tacn representing 1,4,7-triazacyclononane
.
oxide
compound is composed of a central Mn(IV)4O4 cube surrounded by a ring of 8 Mn(III) units connected through bridging oxo ligand
s. In addition, it has 16 acetate
and 4 water
ligands.
It was known in 2006 that the "deliberate structural distortion of a Mn6 compound via the use of a bulky salicylaldoxime
derivative switches the intra-triangular magnetic exchange from antiferromagnetic to ferromagnetic resulting in an S
= 12
ground state
.
A record magnetization was reported in 2007 for a compound related to MnAc12 ([Mn(III)
6O2(sao)6(O2CPh)2(EtOH)4]) with S = 12, D = -0.43 cm−1 and hence U = 62 cm−1 or 86 K at a blocking temperature of 4.3 K. This was accomplished by replacing acetate ligands by the bulkier salicylaldoxime
thus distorting the manganese ligand sphere. It is prepared by mixing the perchlorate
of manganese, the sodium salt of benzoic acid
, a salicylaldoxime
derivate and tetramethylammonium hydroxide
in water and collecting the filtrate.
times temperature
) with decreasing temperature, and can be characterized by a shift both in position and intensity of the a.c. magnetic susceptibility.
Single-molecule magnets represent a molecular approach to nanomagnets (nanoscale magnetic particles). In addition, single-molecule magnets have provided physicists with useful test-beds for the study of quantum mechanics
. Macroscopic quantum tunneling of the magnetization was first observed in Mn12O12, characterized by evenly-spaced steps in the hysteresis curve. The periodic quenching of this tunneling rate in the compound Fe8 has been observed and explained with geometric phase
s.
Due to the typically large, bi-stable spin anisotropy
, single-molecule magnets promise the realization of perhaps the smallest practical unit for magnetic memory
, and thus are possible building blocks for a quantum computer
. Consequently, many groups have devoted great efforts into synthesis of additional single molecule magnets; however, the Mn12O12 complex and analogous complexes remain the canonical single molecule magnet with a 50 cm−1 spin anisotropy.
The spin anisotropy manifests itself as an energy barrier that spins must overcome when they switch from parallel alignment to antiparallel alignment. This barrier (U) is defined as:

where S is the dimensionless total spin state and D the zero-field splitting parameter (in cm−1); D can be negative but only its absolute value
is considered in the equation. The barrier U is generally reported in cm−1 units or in units of Kelvin
(see: electronvolt
). The higher the barrier the longer a material remains magnetized and a high barrier is obtained when the molecule contains many unpaired electrons and when its zero field splitting value is large. For example, the MnAc12 cluster the spin state is 10 (involving 20 unpaired electrons) and D = -0.5 cm−1 resulting in a barrier of 50 cm−1 (equivalent to 60 K
)..
The effect is also observed by hysteresis
experienced when magnetization is measured in a magnetic field
sweep: on lowering the magnetic field again after reaching the maximum magnetization the magnetization remains at high levels and it requires a reversed field to bring magnetization back to zero.
Recently, it has been has been reported that the energy barrier, U, is slightly dependent on Mn12 crystal size/morphology, as well as the magnetization relaxation times, which varies as function of particle size and size distributions .
Metalorganics
Metalorganic compounds are a class of chemical compounds that contain metals and organic ligands. Metalorganic compounds are used extensively in materials science in applications such as metalorganic vapour phase epitaxy or sol-gel processing using alkoxides...
, that show superparamagnetic
Superparamagnetism
Superparamagnetism is a form of magnetism, which appears in small ferromagnetic or ferrimagnetic nanoparticles. In sufficiently small nanoparticles, magnetization can randomly flip direction under the influence of temperature. The typical time between two flips is called the Néel relaxation time...
behavior below a certain blocking temperature at the molecular scale. In this temperature range, SMMs exhibit magnetic hysteresis of purely molecular origin . Contrary to conventional bulk magnets and molecule-based magnets, collective long-range magnetic ordering of magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
s is not necessary .
Intramolecular coupling
The magnetic coupling between the spins of the metal ions is mediated via superexchangeSuperexchange
Superexchange is the strong antiferromagnetic coupling between two next-to-nearest neighbor cations through a non-magnetic anion. In this way, it differs from direct exchange in which there is coupling between nearest neighbor cations not involving an intermediary anion...
interactions and can be described by the following isotropic Heisenberg Hamiltonian
Heisenberg model (quantum)
The Heisenberg model is a statistical mechanical model used in the study of critical points and phase transitions of magnetic systems, in which the spin of the magnetic systems are treated quantum mechanically...
:

where
 is the coupling constant between spin i (operator
is the coupling constant between spin i (operator  ) and spin j (operator
) and spin j (operator  ). For positive J the coupling is called ferromagnetic (parallel alignment of spins) and for negative J the coupling is called antiferromagnetic (antiparallel alignment of spins).
). For positive J the coupling is called ferromagnetic (parallel alignment of spins) and for negative J the coupling is called antiferromagnetic (antiparallel alignment of spins).- a high spinSpin (physics)In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
ground stateGround stateThe ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
, - a high zero-field-splittingZero field splittingZero field splitting describes various interactions of the energy levels of an electron spin even in the absence of an applied magnetic field. It is important in the electron spin resonance of biological molecules....
(due to high magnetic anisotropyAnisotropyAnisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties An example of anisotropy is the light...
), and - negligible magnetic interaction between molecules.
The combination of these properties can lead to an energy barrier so that, at low temperatures, the system can be trapped in one of the high-spin energy wells.
"These molecules contain a finite number of interacting spin centers (e.g. paramagnetic ions) and thus provide ideal opportunities to study basic concepts of magnetism
Magnetism
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well...
. Some of them possess magnetic ground states and give rise to hysteresis
Hysteresis
Hysteresis is the dependence of a system not just on its current environment but also on its past. This dependence arises because the system can be in more than one internal state. To predict its future evolution, either its internal state or its history must be known. If a given input alternately...
effects and metastable magnetic phases. They may show quantum tunneling of the magnetization which raises the question of coherent dynamics in such systems. Other types of molecules exhibit pronounced frustration effects, whereas so-called spin crossover
Spin crossover
Spin Crossover , sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a...
substances can switch their magnetic ground state and related properties such as color under irradiation of laser light, pressure or heat. Scientists from various fields – chemistry, physics; theory and experiment – have joined the research on molecular magnetism in order to explore the unprecedented properties of these new compounds."
"Single-molecule magnets (SMMs) have many important advantages over conventional nanoscale magnetic particles composed of metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s, metal alloys or metal oxides. These advantages include uniform size, solubility in organic solvents, and readily alterable peripheral ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s, among others."
"A single molecule magnet is an example of a macroscopic quantum system. [...] If we could detect spin flips in a single atom or molecule, we could use the spin to store information. This would enable us to increase the storage capacity of computer hard disk
Hard disk
A hard disk drive is a non-volatile, random access digital magnetic data storage device. It features rotating rigid platters on a motor-driven spindle within a protective enclosure. Data is magnetically read from and written to the platter by read/write heads that float on a film of air above the...
s. [...] A good starting point for trying to detect spin flips is to find a molecule with a spin of several Bohr magnetons. [An electron has an intrinsic magnetic dipole moment of approximately one Bohr magneton.] There is a very well studied molecular magnet, Mn12-acetate, which has a spin S = 10 (Figure 3). This molecule is a disc-shaped organic molecule in which twelve Mn ions are embedded. Eight of these form a ring, each having a charge of +3 and a spin S = 2. The other four form a tetrahedron
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
, each having a charge of +4 and a spin S = 3/2. The exchange interactions within the molecule are such that the spins of the ring align themselves in opposition to the spins of the tetrahedron, giving the molecule a total net spin S = 10."
Blocking temperature
Measurements take place at very low temperatures. The so-called blocking temperature is defined as the temperature below which the relaxation of the magnetisation becomes slow compared to the time scale of a particular investigation technique. A molecule magnetised at 2 K will keep 40% of its magnetisation after 2 months and by lowering the temperature to 1.5 K this will take 40 years.Future applications
As of 2008 there are many discovered types and potential uses. "Single molecule magnets (SMM) are a class of molecules exhibiting magnetic properties similar to those observed in conventional bulk magnets, but of molecular origin. SMMs have been proposed as potential candidates for several technological applications that require highly controlled thin films and patterns.""The ability of a single molecule to behave like a tiny magnet (single molecular magnets, SMMs) has seen a rapid growth in research over the last few years. SMMs represent the smallest possible magnetic devices and are a controllable, bottom-up approach to nanoscale magnetism. Potential applications of SMMs include quantum computing
Quantum computer
A quantum computer is a device for computation that makes direct use of quantum mechanical phenomena, such as superposition and entanglement, to perform operations on data. Quantum computers are different from traditional computers based on transistors...
, high-density information storage and magnetic refrigeration
Magnetic refrigeration
Magnetic refrigeration is a cooling technology based on the magnetocaloric effect. This technique can be used to attain extremely low temperatures , as well as the ranges used in common refrigerators, depending on the design of the system.The effect was first observed by the German physicist Emil...
."

Hard disk
A hard disk drive is a non-volatile, random access digital magnetic data storage device. It features rotating rigid platters on a motor-driven spindle within a protective enclosure. Data is magnetically read from and written to the platter by read/write heads that float on a film of air above the...
s. [...] A good starting point for trying to detect spin flips is to find a molecule with a spin of several Bohr magnetons. [An electron has an intrinsic magnetic dipole moment of approximately one Bohr magneton.] There is a very well studied molecular magnet, Mn12-acetate, which has a spin S = 10 (Figure 3). This molecule is a disc-shaped organic molecule in which twelve Mn ions are embedded. Eight of these form a ring, each having a charge of +3 and a spin S = 2. The other four form a tetrahedron
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
, each having a charge of +4 and a spin S = 3/2. The exchange interactions within the molecule are such that the spins of the ring align themselves in opposition to the spins of the tetrahedron, giving the molecule a total net spin S = 10."
Types

Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
(Mn) complex having the formula [Mn12O12(OAc)16(H2O)4], where OAc stands for acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
. It has the remarkable property of showing an extremely slow relaxation of their magnetization below a blocking temperature. [Mn12O12(OAc)16(H2O)4]·4H2O·2AcOH which is called "Mn12-acetate" is a common form of this used in research.
"Mn4" is another researched type single-molecule magnet. Three of these are:
- [Mn4(hmp)6(NO3)2(MeCN)2](ClO4)2·2MeCN
- [Mn4(hmp)6(NO3)4]·(MeCN)
- [Mn4(hmp)4(acac)2(MeO)2](ClO4)2·2MeOH
In each of these Mn4 complexes "there is a planar diamond core of MnIII2MnII2 ions. An analysis of the variable-temperature and variable-field magnetization data indicate that all three molecules have intramolecular ferromagnetic coupling and a S = 9 ground state. The presence of a frequency-dependent alternating current susceptibility signal indicates a significant energy barrier between the spin-up and spin-down states for each of these three MnIII2MnII2 complexes."
Single-molecule magnets are also based on iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
clusters because they potentially have large spin states. In addition the biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
ferritin
Ferritin
Ferritin is a ubiquitous intracellular protein that stores iron and releases it in a controlled fashion. The amount of ferritin stored reflects the amount of iron stored. The protein is produced by almost all living organisms, including bacteria, algae and higher plants, and animals...
is also considered a nanomagnet
Nanomagnet
A nanomagnet is a submicrometric system that presents spontaneous magnetic order at zero applied magnetic field .The small size of nanomagnets prevents the formation of magnetic domains...
. In the cluster Fe8Br the cation Fe8 stands for [Fe8O2(OH)12(tacn)6]8+ with tacn representing 1,4,7-triazacyclononane
1,4,7-Triazacyclononane
1,4,7-Triazacyclononane, known as "TACN" which is pronounced "tack-en," is a cyclic organic compound with the formula C6H123. TACN is derived, formally speaking, from cyclononane by replacing three equidistant CH2 groups with NH groups. TACN is one of the oligomers derived from aziridine, C2H4NH...
.
History
Although the term "single-molecule magnet" was first employed by David Hendrickson, a chemist at the University of California, San Diego and George Christou (Indiana University) in 1996, the first single-molecule magnet reported dates back to 1991. The European researchers discovered that a Mn12O12(MeCO2)16(H2O)4 complex (Mn12Ac16) first synthesized in 1980 exhibits slow relaxation of the magnetization at low temperatures. This manganeseManganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
compound is composed of a central Mn(IV)4O4 cube surrounded by a ring of 8 Mn(III) units connected through bridging oxo ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s. In addition, it has 16 acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
and 4 water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
ligands.
It was known in 2006 that the "deliberate structural distortion of a Mn6 compound via the use of a bulky salicylaldoxime
Salicylaldoxime
Salicylaldoxime is a chemical compound described by the formula C6H4CH=NOH-2-OH. It is the oxime of salicylaldehyde. This crystalline solid is a chelator and sometimes used in the analysis of samples containing transition metal ions, with which it often forms brightly-coloured coordination...
derivative switches the intra-triangular magnetic exchange from antiferromagnetic to ferromagnetic resulting in an S
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
= 12
Quantum number
Quantum numbers describe values of conserved quantities in the dynamics of the quantum system. Perhaps the most peculiar aspect of quantum mechanics is the quantization of observable quantities. This is distinguished from classical mechanics where the values can range continuously...
ground state
Stationary state
In quantum mechanics, a stationary state is an eigenvector of the Hamiltonian, implying the probability density associated with the wavefunction is independent of time . This corresponds to a quantum state with a single definite energy...
.
A record magnetization was reported in 2007 for a compound related to MnAc12 ([Mn(III)
6O2(sao)6(O2CPh)2(EtOH)4]) with S = 12, D = -0.43 cm−1 and hence U = 62 cm−1 or 86 K at a blocking temperature of 4.3 K. This was accomplished by replacing acetate ligands by the bulkier salicylaldoxime
Salicylaldoxime
Salicylaldoxime is a chemical compound described by the formula C6H4CH=NOH-2-OH. It is the oxime of salicylaldehyde. This crystalline solid is a chelator and sometimes used in the analysis of samples containing transition metal ions, with which it often forms brightly-coloured coordination...
thus distorting the manganese ligand sphere. It is prepared by mixing the perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
of manganese, the sodium salt of benzoic acid
Benzoic acid
Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis...
, a salicylaldoxime
Salicylaldoxime
Salicylaldoxime is a chemical compound described by the formula C6H4CH=NOH-2-OH. It is the oxime of salicylaldehyde. This crystalline solid is a chelator and sometimes used in the analysis of samples containing transition metal ions, with which it often forms brightly-coloured coordination...
derivate and tetramethylammonium hydroxide
Tetramethylammonium hydroxide
Tetramethylammonium hydroxide is a quaternary ammonium salt with the molecular formula 4NOH. It is used as an anisotropic etchant of silicon. It is also used as a basic solvent in the development of acidic photoresist in the photolithography process. Since it is a phase transfer catalyst, it is...
in water and collecting the filtrate.
Detailed behavior
Molecular magnets exhibit an increasing product (magnetic susceptibilityMagnetic susceptibility
In electromagnetism, the magnetic susceptibility \chi_m is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field...
times temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
) with decreasing temperature, and can be characterized by a shift both in position and intensity of the a.c. magnetic susceptibility.
Single-molecule magnets represent a molecular approach to nanomagnets (nanoscale magnetic particles). In addition, single-molecule magnets have provided physicists with useful test-beds for the study of quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
. Macroscopic quantum tunneling of the magnetization was first observed in Mn12O12, characterized by evenly-spaced steps in the hysteresis curve. The periodic quenching of this tunneling rate in the compound Fe8 has been observed and explained with geometric phase
Geometric phase
In classical and quantum mechanics, the geometric phase, Pancharatnam–Berry phase , Pancharatnam phase or most commonly Berry phase, is a phase acquired over...
s.
Due to the typically large, bi-stable spin anisotropy
Anisotropy
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties An example of anisotropy is the light...
, single-molecule magnets promise the realization of perhaps the smallest practical unit for magnetic memory
Magnetic memory
Magnetic memory may refer to:* Magnetic storage, the storage of data on a magnetized medium* Remanence, or residual magnetization, the magnetization left behind in a ferromagnet after an external magnetic field is removed...
, and thus are possible building blocks for a quantum computer
Quantum computer
A quantum computer is a device for computation that makes direct use of quantum mechanical phenomena, such as superposition and entanglement, to perform operations on data. Quantum computers are different from traditional computers based on transistors...
. Consequently, many groups have devoted great efforts into synthesis of additional single molecule magnets; however, the Mn12O12 complex and analogous complexes remain the canonical single molecule magnet with a 50 cm−1 spin anisotropy.
The spin anisotropy manifests itself as an energy barrier that spins must overcome when they switch from parallel alignment to antiparallel alignment. This barrier (U) is defined as:

where S is the dimensionless total spin state and D the zero-field splitting parameter (in cm−1); D can be negative but only its absolute value
Absolute value
In mathematics, the absolute value |a| of a real number a is the numerical value of a without regard to its sign. So, for example, the absolute value of 3 is 3, and the absolute value of -3 is also 3...
is considered in the equation. The barrier U is generally reported in cm−1 units or in units of Kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
(see: electronvolt
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
). The higher the barrier the longer a material remains magnetized and a high barrier is obtained when the molecule contains many unpaired electrons and when its zero field splitting value is large. For example, the MnAc12 cluster the spin state is 10 (involving 20 unpaired electrons) and D = -0.5 cm−1 resulting in a barrier of 50 cm−1 (equivalent to 60 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
)..
The effect is also observed by hysteresis
Hysteresis
Hysteresis is the dependence of a system not just on its current environment but also on its past. This dependence arises because the system can be in more than one internal state. To predict its future evolution, either its internal state or its history must be known. If a given input alternately...
experienced when magnetization is measured in a magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
sweep: on lowering the magnetic field again after reaching the maximum magnetization the magnetization remains at high levels and it requires a reversed field to bring magnetization back to zero.
Recently, it has been has been reported that the energy barrier, U, is slightly dependent on Mn12 crystal size/morphology, as well as the magnetization relaxation times, which varies as function of particle size and size distributions .
External links
- European Institute of Molecular Magnetism EIMM
- MAGMANet (Molecular Approach to Nanomagnets and Multifunctional Materials), a Network of centres of Excellence, coordinated by the INSTM – Consorzio Interuniversitario Nazionale per la Scienza e la Tecnologia dei Materiali
- Molecular Magnetism Web, Jürgen Schnack

