Acyloin
Encyclopedia

Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
sharing a common functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
consisting of a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group placed on the α-position of a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group.
Nomenclature
Common types of ketols include:Alpha-ketols have the hydroxyl group adjacent to the keto group.
Beta-ketols have the hydroxyl group at the second carbon from the keto group.
Gamma-ketols have the hydroxyl group at the third carbon from the keto group.
Synthesis of acyloins
Classic organic reactions exist for the synthesis of acyloins.- The acyloin condensationAcyloin condensationAcyloin condensation is a reductive coupling of two carboxylic esters using metallic sodium to yield an α-hydroxyketone, also known as an acyloin....
is a reductive coupling of esters - The benzoin condensationBenzoin condensationThe benzoin condensation is a reaction between two aromatic aldehydes, particularly benzaldehyde. The reaction is catalyzed by a nucleophile such as the cyanide anion or an N-heterocyclic carbene. The reaction product is an aromatic acyloin with benzoin as the parent compound...
is condensation reaction between aldehydes catalyzed by a nucleophileNucleophileA nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the... - Oxidation of carbonylCarbonylIn organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s is possible with molecular oxygen but not selective - Better alternative is oxidation of corresponding silyl enol ethers with mCPBA in the Rubottom oxidationRubottom oxidationThe Rubottom oxidation is the chemical reaction of enolsilanes with m-chloroperoxybenzoic acid to give silyl-protected α-hydroxy ketones.-Reaction mechanism:...
- MoOPH oxidation of carbonylCarbonylIn organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s is a system with molybdenumMolybdenumMolybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
peroxidePeroxideA peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
, pyridinePyridinePyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
and hexamethylphosphoramideHexamethylphosphoramideHexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide having the formula [2N]3PO. This colorless liquid is a useful polar aprotic solvent and additive in organic synthesis.-Structure and reactivity:...
.
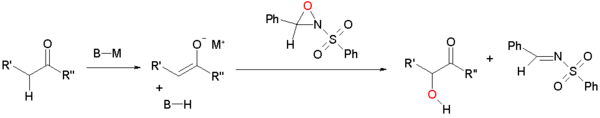
Enolate oxidation by sulfonyloxaziridines
Enolates can be oxidized by sulfonyloxaziridinesOxaziridine
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon.-History:Oxaziridine derivatives were first synthesized in the mid 1950s by Emmons and subsequently by Krimm and Horner and Jürgens...
. The enolate reacts by nucleophilic displacement at the electron deficient oxygen of the oxaziridine
Oxaziridine
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon.-History:Oxaziridine derivatives were first synthesized in the mid 1950s by Emmons and subsequently by Krimm and Horner and Jürgens...
ring.
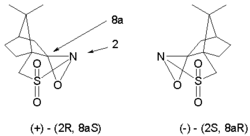
This reaction type is extended to asymmetric synthesis by the use of chiral oxaziridine
Oxaziridine
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon.-History:Oxaziridine derivatives were first synthesized in the mid 1950s by Emmons and subsequently by Krimm and Horner and Jürgens...
s derived from camphor
Camphor
Camphor is a waxy, white or transparent solid with a strong, aromatic odor. It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel , a large evergreen tree found in Asia and also of Dryobalanops aromatica, a giant of the Bornean forests...
(camphorsulfonyl oxaziridine). Each isomer gives exclusive access to one of the two possible enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s. This modification is applied in the Holton Taxol total synthesis
Holton Taxol total synthesis
The Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
.
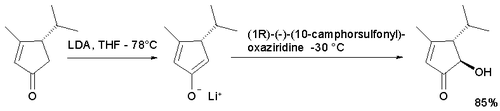
In the enolate oxidation of the cyclopentaenone below with either camphor enantiomer the trans isomer is obtained because access for the hydroxyl group in the cis position is limited. The use of the standard oxaziridine did not result in an acyloin.
Reactions of acyloins
- Reduction of acyloins give diolDiolA diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s. - Oxidation of acyloins give diones.
- Some acyloins rearrange with positions swapped under the influence of base in the Lobry–de Bruyn–van Ekenstein transformation
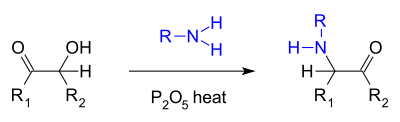
- A similar reaction is the so-called Voight amination where an acyloin reacts with a primary amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
and phosphorus pentoxidePhosphorus pentoxidePhosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
to an alpha-keto amine :
- Indole synthesis, compare Bischler-MöhlauBischler-Möhlau indole synthesisThe Bischler-Möhlau indole synthesis is a chemical reaction that forms a 2-aryl-indole from an α-bromo-acetophenone and excess aniline.In spite of its long history, this classical reaction has received relatively little attention in comparison with other methods for indole synthesis, perhaps owing...
- Indole synthesis, compare Bischler-Möhlau