Organoiron chemistry
Encyclopedia
Organoiron chemistry is the chemistry
of organometallic compounds containing a carbon
-to-iron
chemical bond
. Organoiron compounds are relevant in organic synthesis
as reagent
s such as iron pentacarbonyl
, diiron nonacarbonyl
and disodium tetracarbonylferrate
. Iron adopts oxidation states from Fe(-II) through to Fe(IV). Although iron is generally less active in many catalytic applications, it is less expensive and "greener
" than other metals. Organoiron compounds feature a wide range of ligand
s that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphine
s, carbon monoxide
, and cyclopentadienyl
, but hard ligands
such as amines are employed as well.
, diiron nonacarbonyl
, and triiron dodecacarbonyl
. One or more carbonyl ligands in these compounds can be replaced by a variety of other ligands (dienes, phosphines).
Iron carbonyls have been used in stoichiometric carbonylation
reactions, e.g. for the conversion of alkyl bromides to aldehydes. Disodium tetracarbonylferrate
, "Collman's Reagent," can be alkylated followed by carbonylation to give the acyl derivatives that undergo protonolysis to afford aldehydes:
Similar iron acyls can be accessed by treating iron pentacarbonyl with organolithium compounds:
In this case, the carbanion attacks a CO ligand. In a complementary reaction, Collman's reagent can be used to convert acyl chlorides to aldehydes. Similar reactions can be achieved with [HFe(CO)4]- salts.
, norbornadiene
and cyclooctadiene
, but even cyclobutadiene
can be stabilized. In the complex with butadiene, the diene adopts a cis-conformation. Iron carbonyls are used as a protective group for dienes in hydrogenation
s and Diels-Alder reactions. Cyclobutadieneiron tricarbonyl
is prepared from 3,4-dichlorocyclobutene and Fe2(CO)9.
Cyclohexadienes, many derived from Birch reduction
of aromatic compounds, form derivatives (diene)Fe(CO)3. The affinity of the Fe(CO)3 unit for conjugated dienes is manifested in the ability of iron carbonyls catalyse the isomerisation
s of 1,5-cyclooctadiene
to 1,3-cyclooctadiene
. Cyclohexadiene complexes undergo hydride abstraction to give cyclohexadienyl cations, which add nucleophiles.
The enone complex (benzylideneacetone)iron tricarbonyl
serves as a source of the Fe(CO)3 subunit and is employed to prepare other derivatives. It is used complementarily to Fe29.
Alkynes form many compounds with upon reaction with iron carbonyls. These include cyclobutadiene derivatives, ferroles" of the formula Fe2(C4R4)(CO)6, as well as cyclopentadienone and cyclobutadiene derivatives.
, a very stable that foreshadowed the synthesis of many related sandwich compound
s. Ferrocene
is formed by reaction of sodium cyclopentadienyl with iron chloride
:
Ferrocene displays diverse reactivity localized on the cyclopentadienyl ligands, including Friedel-Crafts reactions and lithation. Ferrocene is also an structurally unusual scaffold as illustrated by the popularity of ligands such as 1,1'-bis(diphenylphosphino)ferrocene
, which are useful in catalysis. Treatment of ferrocene with aluminium trichloride and benzene gives the cation [CpFe(C6H6)]+. Oxidation of ferrocene gives the blue 17e species ferrocenium
.
to give the cyclopentadienyl complex
cyclopentadienyliron dicarbonyl dimer
([FeCp(CO)2]2). Reduction of this species with sodium gives "NaFp" (Fp = [FeCp(CO)2]-), a potent nucleophile
and precursor to many derivatives of the type CpFe(CO)2R. The derivative [FpCH2S(CH3)2]+ has been used in cyclopropanations. FpAcyl compounds are prochiral
, and studies have exploited the chiral derivatives CpFe(PPh3)(CO)acyl. Pyrolysis of Fp2 gives the cuboidal cluster [FeCp(CO)]4.
, azulene
, cyclooctatetraene
(COT), and bullvalene
. The compound Fe(COT)2 is well known , Fe3(COT)3 was described in 2009 as the reaction product of Fe(COT2 with a catalytic amount of an persistent carbene
. It can be regarded as an organic version of triiron dodecacarbonyl
and to a lesser extent amine/imine ligands. Complexes of the type FeX2(diphosphine
)2 figure prominently in this area, provided early examples of C-H bond activation
, dihydrogen complex
es, and dinitrogen complexes. Complexes derived from Schiff bases are active catalysts for olefin polymerization.
s is overshadowed by the related chemistries of cobalt
and nickel. Some main categories are:
, organoiron species are found at the active sites of the three hydrogenase
enzymes as well as carbon monoxide dehydrogenase.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
of organometallic compounds containing a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-to-iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. Organoiron compounds are relevant in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
as reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s such as iron pentacarbonyl
Iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
, diiron nonacarbonyl
Diiron nonacarbonyl
Diiron nonacarbonyl is an inorganic compound with the formula Fe29. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe than Fe5 and less dangerous to handle because it is nonvolatile...
and disodium tetracarbonylferrate
Disodium tetracarbonylferrate
Disodium tetracarbonylferrate is the chemical compound with the formula Na2[Fe4]. This oxygen-sensitive colourless solid is employed in organic synthesis", mainly to synthesise aldehydes. It is commonly used with dioxane complexed to the sodium cation, this dioxane solvate being known as Collman's...
. Iron adopts oxidation states from Fe(-II) through to Fe(IV). Although iron is generally less active in many catalytic applications, it is less expensive and "greener
Green chemistry
Green chemistry, also called sustainable chemistry, is a philosophy of chemical research and engineering that encourages the design of products and processes that minimize the use and generation of hazardous substances...
" than other metals. Organoiron compounds feature a wide range of ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
s, carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, and cyclopentadienyl
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
, but hard ligands
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
such as amines are employed as well.
The binary carbonyls and their anions
Important iron carbonyls are the three neutral binary carbonyls, iron pentacarbonylIron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
, diiron nonacarbonyl
Diiron nonacarbonyl
Diiron nonacarbonyl is an inorganic compound with the formula Fe29. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe than Fe5 and less dangerous to handle because it is nonvolatile...
, and triiron dodecacarbonyl
Triiron dodecacarbonyl
Triiron dodecarbonyl is the chemical compound with the formula Fe312. It was one of the first metal carbonyl clusters synthesized. It is a more reactive source of iron than is iron pentacarbonyl.-General properties:...
. One or more carbonyl ligands in these compounds can be replaced by a variety of other ligands (dienes, phosphines).
Iron carbonyls have been used in stoichiometric carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
reactions, e.g. for the conversion of alkyl bromides to aldehydes. Disodium tetracarbonylferrate
Disodium tetracarbonylferrate
Disodium tetracarbonylferrate is the chemical compound with the formula Na2[Fe4]. This oxygen-sensitive colourless solid is employed in organic synthesis", mainly to synthesise aldehydes. It is commonly used with dioxane complexed to the sodium cation, this dioxane solvate being known as Collman's...
, "Collman's Reagent," can be alkylated followed by carbonylation to give the acyl derivatives that undergo protonolysis to afford aldehydes:
- LiFe(CO)4(C(O)R) + H+ → RCHO (+ iron containing products)
Similar iron acyls can be accessed by treating iron pentacarbonyl with organolithium compounds:
- ArLi + Fe(CO)5 → LiFe(CO)4C(O)R
In this case, the carbanion attacks a CO ligand. In a complementary reaction, Collman's reagent can be used to convert acyl chlorides to aldehydes. Similar reactions can be achieved with [HFe(CO)4]- salts.
(Diene)Fe(CO)3 derivatives
Iron diene complexes are usually prepared from Fe(CO)5 or Fe2(CO)9. Derivatives are known for common dienes are cyclohexadieneCyclohexadiene
-See also:* Benzene or its theoretical isomer 1,3,5-Cyclohexatriene* Cyclohexene...
, norbornadiene
Norbornadiene
Norbornadiene is an organic compound. This bicyclic hydrocarbon is the most stable diolefin derived from the norbornane and norbornene. Norbornadiene is primarily of interest as a ligand in homogeneous catalysis, but it has been heavily studied due to its high reactivity and distinctive...
and cyclooctadiene
Cyclooctadiene
A cyclooctadiene is a cyclic diene with the formula C8H12. Focusing only on cis derivatives, four isomers are possible: 1,2, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition...
, but even cyclobutadiene
Cyclobutadiene
Cyclobutadiene is the smallest [n]-annulene , an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula 44 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel...
can be stabilized. In the complex with butadiene, the diene adopts a cis-conformation. Iron carbonyls are used as a protective group for dienes in hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
s and Diels-Alder reactions. Cyclobutadieneiron tricarbonyl
Cyclobutadieneiron tricarbonyl
Cyclobutadieneiron tricarbonyl or Fe3 is an organometallic complex of cyclobutadiene and an iron metal carbonyl. The chemical compound is used in organic chemistry as a precursor for cyclobutadiene...
is prepared from 3,4-dichlorocyclobutene and Fe2(CO)9.
Cyclohexadienes, many derived from Birch reduction
Birch reduction
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
of aromatic compounds, form derivatives (diene)Fe(CO)3. The affinity of the Fe(CO)3 unit for conjugated dienes is manifested in the ability of iron carbonyls catalyse the isomerisation
Isomerisation
In chemistry isomerisation is the process by which one molecule is transformed into another molecule which has exactly the same atoms, but the atoms are rearranged e.g. A-B-C → B-A-C . In some molecules and under some conditions, isomerisation occurs spontaneously...
s of 1,5-cyclooctadiene
1,5-Cyclooctadiene
1,5-Cyclooctadiene is the organic compound with the chemical formula C8H12. Generally abbreviated COD, this diene is a useful precursor to other organic compounds and serves as a ligand in organometallic chemistry.- Synthesis :...
to 1,3-cyclooctadiene
Cyclooctadiene
A cyclooctadiene is a cyclic diene with the formula C8H12. Focusing only on cis derivatives, four isomers are possible: 1,2, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition...
. Cyclohexadiene complexes undergo hydride abstraction to give cyclohexadienyl cations, which add nucleophiles.
The enone complex (benzylideneacetone)iron tricarbonyl
(Benzylideneacetone)iron tricarbonyl
The organometallic compound iron tricarbonyl is a widely employed reagent for transferring the Fe3 unit to other organic molecules. This red-colored compound is commonly abbreviated Fe3. It is prepared by the reaction of Fe29 with benzylideneacetone, typically in refluxing diethyl ether...
serves as a source of the Fe(CO)3 subunit and is employed to prepare other derivatives. It is used complementarily to Fe29.
Alkynes form many compounds with upon reaction with iron carbonyls. These include cyclobutadiene derivatives, ferroles" of the formula Fe2(C4R4)(CO)6, as well as cyclopentadienone and cyclobutadiene derivatives.
Sulfur and phosphorus derivatives
Complexes of the type Fe2(SR)2(CO)6 and Fe2(PR2)2(CO)6 form, usually by the reaction of thiols and secondary phosphines with iron carbonyls. The thiolates can also be obtained from the tetrahedrane Fe2S2(CO)6.Cyclopentadienyl derivatives, including ferrocenes
Ferrocene and its derivatives
The rapid growth of organometallic chemistry in the 20th Century can be traced to the discovery of ferroceneFerrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
, a very stable that foreshadowed the synthesis of many related sandwich compound
Sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives...
s. Ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
is formed by reaction of sodium cyclopentadienyl with iron chloride
Iron chloride
Iron chloride can refer to* Iron chloride , FeCl2* Iron chloride , FeCl3...
:
- 2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
Ferrocene displays diverse reactivity localized on the cyclopentadienyl ligands, including Friedel-Crafts reactions and lithation. Ferrocene is also an structurally unusual scaffold as illustrated by the popularity of ligands such as 1,1'-bis(diphenylphosphino)ferrocene
1,1'-Bis(diphenylphosphino)ferrocene
1,1'-Bisferrocene, commonly abbreviated dppf, is a substituted phosphine commonly used, as are other phosphines, as a ligand in organometallic chemistry...
, which are useful in catalysis. Treatment of ferrocene with aluminium trichloride and benzene gives the cation [CpFe(C6H6)]+. Oxidation of ferrocene gives the blue 17e species ferrocenium
Ferrocenium tetrafluoroborate
Ferrocenium tetrafluoroborate is an organometallic compound with the formula [Fe2]BF4. This salt is composed of the cation [Fe2]+ and the tetrafluoroborate anion . The related hexafluorophosphate is also a popular reagent with similar properties. The cation is often abbreviated Fc+...
.
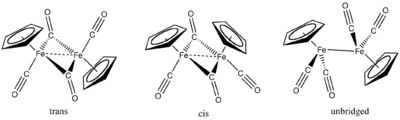
Fp2 and its derivatives
Fe(CO)5 reacts with dicyclopentadieneDicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white crystalline solid with a camphor-like odor. Its energy density is 10,975 Wh/l....
to give the cyclopentadienyl complex
Cyclopentadienyl complex
A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic...
cyclopentadienyliron dicarbonyl dimer
Cyclopentadienyliron dicarbonyl dimer
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula 2Fe24, also abbreviated Cp2Fe24. It is called Fp2 or "fip dimer." It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but...
([FeCp(CO)2]2). Reduction of this species with sodium gives "NaFp" (Fp = [FeCp(CO)2]-), a potent nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
and precursor to many derivatives of the type CpFe(CO)2R. The derivative [FpCH2S(CH3)2]+ has been used in cyclopropanations. FpAcyl compounds are prochiral
Prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step.If two identical substituents are attached to a sp3-hybridized atom, the descriptors pro-R and pro-S are used to distinguish between the two...
, and studies have exploited the chiral derivatives CpFe(PPh3)(CO)acyl. Pyrolysis of Fp2 gives the cuboidal cluster [FeCp(CO)]4.
Polyhapto organic ligands
Stable complexes with iron with and without CO ligands are known for a wide variety of polyunsaturated hydrocarbons, e.g. cycloheptatrieneCycloheptatriene
Cycloheptatriene is an organic compound with the formula C7H8. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and as a building block in organic synthesis...
, azulene
Azulene
Azulene is an organic compound and an isomer of naphthalene. Whereas naphthalene is colourless, azulene is dark blue. Its name is derived from the Spanish word azul, meaning "blue"...
, cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
(COT), and bullvalene
Bullvalene
Bullvalene is a hydrocarbon with the chemical formula C10H10 with the unusual property that the chemical bonds making up the molecule are constantly rearranging as in fluxional molecules...
. The compound Fe(COT)2 is well known , Fe3(COT)3 was described in 2009 as the reaction product of Fe(COT2 with a catalytic amount of an persistent carbene
Persistent carbene
A persistent carbene is a type of carbene demonstrating particular stability. The best-known examples are diaminocarbenes with the general formula 2C:, where the 'R's are various functional groups...
. It can be regarded as an organic version of triiron dodecacarbonyl
Triiron dodecacarbonyl
Triiron dodecarbonyl is the chemical compound with the formula Fe312. It was one of the first metal carbonyl clusters synthesized. It is a more reactive source of iron than is iron pentacarbonyl.-General properties:...
Phosphine- and amine-Fe(II) complexes
As for other organometallic compounds, organoiron(II) complexes in the absence of Cp ligands are commonly complemented by tertiary diphosphinesDiphosphines
Diphosphines, sometimes called bisphosphanes are organophosphorus compounds that are used as ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone and are usually chelating...
and to a lesser extent amine/imine ligands. Complexes of the type FeX2(diphosphine
Diphosphines
Diphosphines, sometimes called bisphosphanes are organophosphorus compounds that are used as ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone and are usually chelating...
)2 figure prominently in this area, provided early examples of C-H bond activation
C-H bond activation
Carbon–hydrogen bond activation or C−H activation may be defined as a reaction that cleaves a carbon–hydrogen bond. Often the term is restricted to reactions involving organometallic complexes and proceeding by coordination of a hydrocarbon to the inner-sphere of metal, either via an...
, dihydrogen complex
Dihydrogen complex
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. The prototypical complex is W32. This class of compounds represent intermediates in metal-catalyzed reactions involving hydrogen. Hundreds of dihydrogen complexes have been reported...
es, and dinitrogen complexes. Complexes derived from Schiff bases are active catalysts for olefin polymerization.
Organoiron compounds in organic synthesis and homogeneous catalysis
Because of its low cost and low toxicity of its salts, iron is often employed as a stoichiometric reagent. Iron role as a catalyst in organic reactionOrganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s is overshadowed by the related chemistries of cobalt
Organocobalt chemistry
Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds are investigated for...
and nickel. Some main categories are:
- Addition reactionAddition reactionAn addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
s for example the Aldol reactionAldol reactionThe aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
and Michael reactionMichael reactionThe Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds... - SubstitutionSubstitution reactionIn a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
notably coupling reactions. Ferric chloride is a well known catalyst in the Friedel–Crafts reaction. Compounds of the type [(η3-allyl)Fe(CO)4+X- are allyl cation synthonSynthonA synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
s in allylic substitution . Likewise the compound Ph(CO2)Fe+(η2-vinyl(OEt))BF4- is a masked vinyl cationVinyl cationA vinyl cation is a positively charged molecule that also contains a vinyl group . Generally the charge is on one of the double-bonded carbons....
. Disodium tetracarbonylferrateDisodium tetracarbonylferrateDisodium tetracarbonylferrate is the chemical compound with the formula Na2[Fe4]. This oxygen-sensitive colourless solid is employed in organic synthesis", mainly to synthesise aldehydes. It is commonly used with dioxane complexed to the sodium cation, this dioxane solvate being known as Collman's...
can be regarded a CO dianion synthon. - CycloadditionCycloadditionA cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s, for example cyclopropanation using CpFe(CO)2CH2S+(CH3)2BF4- . Also Ene reactionEne reactionThe Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the... - HydrogenationHydrogenationHydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
and reduction - isomerization reactions and rearrangement reactions
- Cross-coupling reactions. Iron compounds such as Fe(acacAcacACAC may refer to:* American Council for Accredited Certification, a private, non-profit certifying body* Atlanta Contemporary Art Center, a contemporary art museum in Atlanta* ACAC consortium, a subsidiary of China Aviation Industry Corporation...
)3 catalyze a wide range of cross-coupling reactions with one substrate an arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
or alkyl GrignardGrignardGrignard can be a French last name, or refers to an organic chemical reaction.* Victor Grignard, a French organic chemist.* The Grignard Company a chemical manufacturer.* Grignard reaction, an organic chemical reaction developed by Victor Grignard....
and the other substrate an aryl, alkenyl (vinyl), or acylAcylAn acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
organohalide. In the related Kumada couplingKumada couplingA Kumada coupling or Kumada-Corriu coupling is a cross coupling reaction in organic chemistry between an alkyl or aryl Grignard reagent and an aryl or vinyl halocarbon catalysed by nickel or palladium. This reaction is relevant to organic synthesis because it gives access to styrene derivatives...
the catalysts are based on palladiumPalladiumPalladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
and nickelNickelNickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
.
Biochemistry
In the area of bioorganometallic chemistryBioorganometallic chemistry
Bioorganometallic chemistry is the study of biologically active molecules that contain carbon directly bonded to metals or metalloids. This area straddles the fields of organometallic chemistry, biochemistry, and medicine. It is subset of bioinorganic chemistry. Naturally occurring...
, organoiron species are found at the active sites of the three hydrogenase
Hydrogenase
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism....
enzymes as well as carbon monoxide dehydrogenase.
See also
- Chemical bonds of carbon with other elements in the periodic table: