C-H bond activation
Encyclopedia
Carbon–hydrogen bond activation or C−H activation may be defined as a reaction that cleaves a carbon–hydrogen bond. Often the term is restricted to reactions involving organometallic complexes and proceeding by coordination of a hydrocarbon
to the inner-sphere of metal, either via an intermediate “alkane or arene complex” or as a transition state
leading to a "M−C" intermediate. Important to this definition is the requirement that during the C−H cleavage event the hydrocarbyl species remains associated in the inner-sphere and under the influence of “M”.
Theoretical studies as well as experimental investigations indicate that C−H bonds, which are traditionally considered unreactive, can be cleaved by coordination. Much research effort has been devoted to the design and synthesis of new reagents and catalysts that can affect CH activation. A significant driver for this type of research is the prospect that C−H activation could enable the conversion of cheap and abundant alkanes into valuable functionalized
organic compounds.
who in 1902 reported that benzene
with mercury(II) acetate
(See: organomercury
), but some scholars do not view this reaction as being truly C−H activation. As observed Goldman & Goldberg C−H activation resembles aspects of H−H activation: both can be achieved by electrophilic or oxidative addition.
The first true C−H activation reaction was reported by Joseph Chatt
in 1965 with insertion of a ruthenium
atom ligated to dmpe
in the C−H bond of naphthalene
. In 1969 A.E. Shilov
reported that potassium tetrachloroplatinate
induced isotope scrambling between methane
and heavy water
. The pathway was proposed to involve binding of methane to Pt(II). In 1972 the Shilov group was able to produce methanol
and methyl chloride in a similar reaction of stoichiometric potassium tetrachloroplatinate
, catalytic potassium hexachloroplatinate
, methane and water. As Shilov worked and published in Cold War Soviet Union his work was largely ignored by Western scientists. This so-called Shilov system
is today one of the few true catalytic systems for mild alkane
functionalizations.
On the other side of the spectrum, oxidative addition, M. L. H. Green
in 1970 reported on the photochemical insertion of tungsten
(as a Cp2WH2 complex) in a benzene
C−H bond and George M. Whitesides
in 1979 was the first to carry out an intramolecular
aliphatic C−H activation
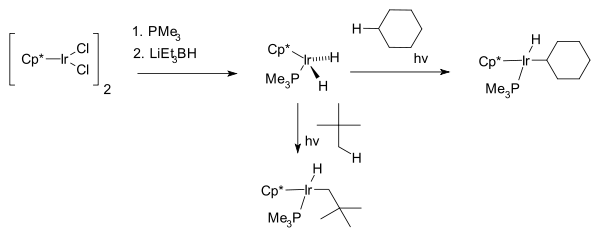
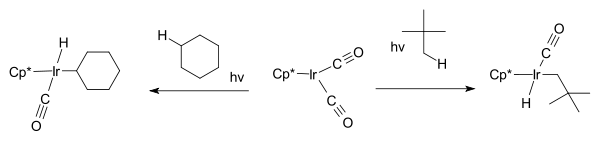
The next breakthrough was reported independently by two research groups 1982, by R. G. Bergman with the first photochemical C−H activation of completely saturated hydrocarbons cyclohexane
and neopentane
forming the hydridoalkylmetal complex Cp*Ir(PMe3)H(C6H5) where Cp* is a pentamethylcyclopentadienyl ligand.
W.A.G. Graham found that the same hydrocarbons react with Cp*Ir(CO)2 to afford iridium hydrido complexes.
The first part of the reaction; the iridium complex reacting with the cycloalkane is an example of oxidative addition where the hydrogen is removed from the alkane and oxidatively added to the metal.
J.F. Hartwig reported a highly regioselective arene and alkane borylation catalyzed by a rhodium complex in 1999 and 2000. In the case of alkanes, exclusive terminal functionalization was observed.
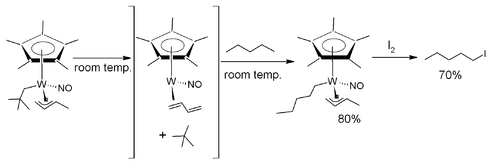
is selectively converted to the halocarbon
1-iodopentane with the aid of a tungsten
complex.
The tungsten complex is fitted with a pentamethylcyclopentadienyl, a nitrosyl
, a 3η
1-butene
and a neopentanyl CH2C(CH3)3 ligand. It is thermally unstable and when dissolved in pentane at room temperature
it loses neopentane
(gains a proton) and coordinates with a pentane ligand (loses a proton). This proton exchange proceeds via a 16 electron
intermediate with a butadiene ligand after beta elimination. In a separate step iodine
is added at −60°C and 1-iodopentane is released.
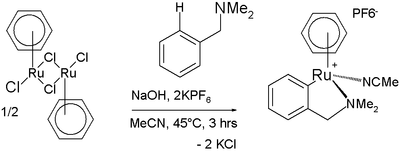
Arene C−H bonds can also be activated by metal complexes despite being fairly unreactive. One manifestation is found in the Murai olefin coupling. In one reaction a ruthenium
complex reacts with N,N-dimethylbenzylamine in a cyclometalation also involving C−H activation :
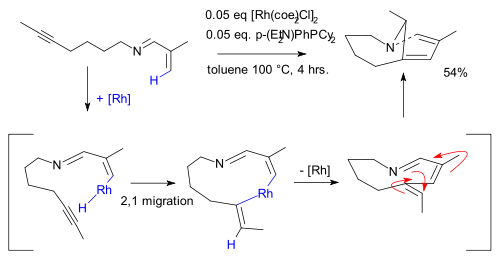
An alkene C−H bond activation with a rhodium
catalyst is demonstrated in the synthesis of this strained bicyclic enamine :
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
to the inner-sphere of metal, either via an intermediate “alkane or arene complex” or as a transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
leading to a "M−C" intermediate. Important to this definition is the requirement that during the C−H cleavage event the hydrocarbyl species remains associated in the inner-sphere and under the influence of “M”.
Theoretical studies as well as experimental investigations indicate that C−H bonds, which are traditionally considered unreactive, can be cleaved by coordination. Much research effort has been devoted to the design and synthesis of new reagents and catalysts that can affect CH activation. A significant driver for this type of research is the prospect that C−H activation could enable the conversion of cheap and abundant alkanes into valuable functionalized
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
organic compounds.
Historic overview
The first C−H activation reaction is often attributed to Otto DimrothOtto Dimroth
Otto Dimroth was a German chemist. He is known for the Dimroth rearrangement, as well as a type of condenser with an internal double spiral, the Dimroth condenser....
who in 1902 reported that benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
with mercury(II) acetate
Mercury(II) acetate
Mercury acetate is the chemical compound with the formula Hg2. Commonly abbreviated Hg2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.-Structure:...
(See: organomercury
Organomercury
Organomercury refers to the group of organometallic compounds that contain mercury. Typically the Hg-C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury cation, CH3Hg+; ethylmercury cation, C2H5Hg+; dimethylmercury, 2Hg,...
), but some scholars do not view this reaction as being truly C−H activation. As observed Goldman & Goldberg C−H activation resembles aspects of H−H activation: both can be achieved by electrophilic or oxidative addition.
The first true C−H activation reaction was reported by Joseph Chatt
Joseph Chatt
Joseph Chatt, CBE FRS was a renowned researcher in the area of inorganic and organometallic chemistry. His name is associated with the description of the pi-bond between transition metals and alkenes, the so-called Dewar-Chatt-Duncanson model.Chatt received his Ph.D. at the University of...
in 1965 with insertion of a ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
atom ligated to dmpe
1,2-Bis(dimethylphosphino)ethane
1,2-Bisethane is a diphosphine ligand in coordination chemistry. It can be synthesised by the reaction of methylmagnesium iodide with 1,2-bisethane:...
in the C−H bond of naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
. In 1969 A.E. Shilov
Alexander E. Shilov
Alexander E. Shilov was born in Ivanovo, Russia. He studied Chemistry in Kiev and received his diploma degee in 1952 from Kiev State University. In 1952-1955 he was working with Nobel Laureate Nikolay Semyonov toward his Ph.D. at the Academy of Sciences in Moscow...
reported that potassium tetrachloroplatinate
Potassium tetrachloroplatinate
Potassium tetrachloroplatinate is the chemical compound with the formula K2PtCl4. This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42-...
induced isotope scrambling between methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
and heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
. The pathway was proposed to involve binding of methane to Pt(II). In 1972 the Shilov group was able to produce methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
and methyl chloride in a similar reaction of stoichiometric potassium tetrachloroplatinate
Potassium tetrachloroplatinate
Potassium tetrachloroplatinate is the chemical compound with the formula K2PtCl4. This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42-...
, catalytic potassium hexachloroplatinate
Potassium hexachloroplatinate
Potassium hexachloroplatinate, an inorganic compound, is an example of a comparatively insoluble potassium salt.The precipitation of this compound from solutions of hexachloroplatinic acid was formerly used for the determination of potassium by gravimetry....
, methane and water. As Shilov worked and published in Cold War Soviet Union his work was largely ignored by Western scientists. This so-called Shilov system
Shilov system
The Shilov system is a classic example of catalytic C-H bond activation and oxidation which preferentially activates stronger C-H bonds over weaker C-H bonds for an overall partial oxidation. - Overview :The Shilov system was discovered by Alexander E...
is today one of the few true catalytic systems for mild alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
functionalizations.
On the other side of the spectrum, oxidative addition, M. L. H. Green
Malcolm Green (chemist)
Malcolm Green also known as M. L. H. Green is a British Emeritus Professor of Inorganic Chemistry.Born in Eastleigh, Hampshire, he received his BSc degree from Acton Technical College in 1956 and his PhD from Imperial College of Science and Technology in 1959 under the supervision of Professor...
in 1970 reported on the photochemical insertion of tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
(as a Cp2WH2 complex) in a benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
C−H bond and George M. Whitesides
George M. Whitesides
George M. Whitesides is an American chemist and professor of chemistry at Harvard University. He is best known for his work in the areas of NMR spectroscopy, organometallic chemistry, molecular self-assembly, soft lithography, microfabrication, microfluidics, and nanotechnology...
in 1979 was the first to carry out an intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
aliphatic C−H activation
The next breakthrough was reported independently by two research groups 1982, by R. G. Bergman with the first photochemical C−H activation of completely saturated hydrocarbons cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
and neopentane
Neopentane
Neopentane, also called dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is an extremely flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher...
forming the hydridoalkylmetal complex Cp*Ir(PMe3)H(C6H5) where Cp* is a pentamethylcyclopentadienyl ligand.
W.A.G. Graham found that the same hydrocarbons react with Cp*Ir(CO)2 to afford iridium hydrido complexes.
The first part of the reaction; the iridium complex reacting with the cycloalkane is an example of oxidative addition where the hydrogen is removed from the alkane and oxidatively added to the metal.
J.F. Hartwig reported a highly regioselective arene and alkane borylation catalyzed by a rhodium complex in 1999 and 2000. In the case of alkanes, exclusive terminal functionalization was observed.
Scope
In one study the alkane pentanePentane
Pentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called...
is selectively converted to the halocarbon
Halocarbon
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds...
1-iodopentane with the aid of a tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
complex.
The tungsten complex is fitted with a pentamethylcyclopentadienyl, a nitrosyl
Metal nitrosyl
Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.-Bonding and structure:...
, a 3η
Hapticity
The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to...
1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
and a neopentanyl CH2C(CH3)3 ligand. It is thermally unstable and when dissolved in pentane at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
it loses neopentane
Neopentane
Neopentane, also called dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is an extremely flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher...
(gains a proton) and coordinates with a pentane ligand (loses a proton). This proton exchange proceeds via a 16 electron
Electron counting
Electron counting is a formalism used for classifying compounds and for explaining or predicting electronic structure and bonding. Many rules in chemistry rely on electron-counting:...
intermediate with a butadiene ligand after beta elimination. In a separate step iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
is added at −60°C and 1-iodopentane is released.
Arene C−H bonds can also be activated by metal complexes despite being fairly unreactive. One manifestation is found in the Murai olefin coupling. In one reaction a ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
complex reacts with N,N-dimethylbenzylamine in a cyclometalation also involving C−H activation :
An alkene C−H bond activation with a rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
catalyst is demonstrated in the synthesis of this strained bicyclic enamine :
Reaction conditions
Most C-H bond activations proceed under rather harsh reaction conditions (high T, strongly acidic or basic conditions, strong oxidant), significantly lowering their attractiveness. However, more and more quite mild reactions have been developed, significantly expanding the scope of these exciting transformations.Further reading
- “Activation of C−H Bonds by Metal Complexes”, A. E. Shilov, G. B. Shul’pin, Chem. Rev. 1997, 97, 2879–2932.
- “Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes”, A. E. Shilov, G. B. Shul’pin, Kluwer Academic Publishers, Dordrecht/Boston/London, 2000 (552 p) (Springer, ISBN 978-0-7923-6101-5). http://www.springer.com/chemistry/physical+chemistry/book/978-0-7923-6101-5
- “Alkane C−H activation and functionalization with homogeneous transition metal catalysts: a century of progress – a new millennium in prospect”, R. H. Crabtree, J. Chem. Soc., Dalton Trans. 2001, 17, 2437–2450.
- “Organometallic alkane CH activation”, R. H. Crabtree, J. Organometal. Chem. 2004, 689, 4083–4091.
- “Mechanistic Aspects of C−H Activation by Pt Complexes”, M. Lersch, M.Tilset, Chem. Rev. 2005, 105, 2471−2526.
- “Recent Advances in the Platinum-mediated CH Bond Functionalization”, A. N. Vedernikov, Curr. Org. Chem. 2007, 11, 1401−1416.
- “Catalytic C−H functionalization by metalcarbenoid and nitrenoid insertion”, H. M. L. Davies, J. R. Manning, Nature, 2008, 451, 417−424,
- "Mechanisms of C−H bond activation: rich synergy between computation and experiment”, Y. Boutadla, D. L. Davies, S. A. Macgregor, A. I. Poblador-Bahamonde, Dalton Trans. 2009, 5820−5831.
- “C−H Bond Activation in Transition Metal Species from a Computational Perspective”, D. Balcells, E. Clot, O. Eisenstein, Chem. Rev. 2010, 110, 749–823.
- “Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions”, T. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147–1169.
- “Selectivity enhancement in functionalization of C−H bonds: A review”, G. B. Shul’pin, Org. Biomol. Chem. 2010, 8, 4217–4228.