1,5-Cyclooctadiene
Encyclopedia
1,5-Cyclooctadiene is the organic compound
with the chemical formula C8H12. Generally abbreviated COD, this diene
is a useful precursor to other organic compounds and serves as a ligand
in organometallic chemistry
.
in organic chemistry used in hydroborations:
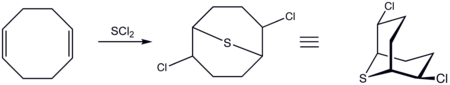
COD adds SCl2 (or similar reagents) to give 2,6-dichloro-9-thiabicyclo[3.3.1]nonane:
The resulting dichloride can be further modified as the di-azide
or di-cyano derivative in a nucleophilic substitution
aided by anchimeric assistance.
Ni(COD)2 is prepared by reduction of anhydrous
nickel acetylacetonate in the presence of the ligand, using triethylaluminium
The related Pt(COD)2 is prepared by a more circuitous route involving the dilithium cyclooctatetraene
:
Extensive work has been reported on complexes of COD, much of which can has been described in volumes 25, 26, and 28 of Inorganic Syntheses
. The platinum complex has been used in many syntheses:
COD complexes are useful as starting materials, one noteworthy example is the reaction:
The product Ni(CO)4 is highly toxic, thus it is advantageous to generate it in the reaction vessel as opposed to being dispensed directly. Other low-valent metal complexes of COD include Mo(COD)(CO)4, [RuCl2(COD)]n, and Fe(COD)(CO)3. COD is an especially important in the coordination chemistry of rhodium(I) and iridium(I), examples being Crabtree's catalyst
and cyclooctadiene rhodium chloride dimer. The square planar complexes [M(COD)2]+ are known (M = Rh, Ir).
and Cope
in 1969 by photoisomerization of the cis compound. Another synthesis (double elimination reaction from a cyclooctane ring) was reported by Huisgen
in 1987. The molecular conformation of (E,E)-COD is twisted rather than chair-like. The compound has been investigated as a click chemistry
mediator.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the chemical formula C8H12. Generally abbreviated COD, this diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
is a useful precursor to other organic compounds and serves as a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
.
Synthesis
1,5-Cyclooctadiene can be prepared by dimerization of butadiene in the presence of a nickel catalyst, a coproduct being vinylcyclohexene. Approximately 10,000 tons were produced in 2005.Organic reactions
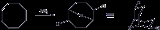
COD reacts with borane to give 9-borabicyclo[3.3.1]nonane, commonly known as 9-BBN, a reagentReagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
in organic chemistry used in hydroborations:
COD adds SCl2 (or similar reagents) to give 2,6-dichloro-9-thiabicyclo[3.3.1]nonane:
The resulting dichloride can be further modified as the di-azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
or di-cyano derivative in a nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
aided by anchimeric assistance.
Metal complexes
1,5-COD typically binds to low-valence metals via both alkene groups. The complex Ni(cod)2 is a precursor to several nickel(0) and Ni(II) complexes. Metal-COD complexes are attractive because they are sufficiently stable to be isolated, often being more robust than related ethylene complexes. The stability of COD complexes is attributable to the chelate effect. The COD ligands are easily displaced by other ligands, such as phosphines.Ni(COD)2 is prepared by reduction of anhydrous
Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
nickel acetylacetonate in the presence of the ligand, using triethylaluminium
Triethylaluminium
Triethylaluminium is an organoaluminium compound. This volatile, colorless liquid is highly pyrophoric, igniting immediately upon exposure to air. It is normally stored in stainless steel containers either as a pure liquid or as a solution in hydrocarbon solvents such as hexane, heptane, or ...
- 1/3 [Ni(C5H7O2)2]3 + 2 COD + 2 Al(C2H5)3 → Ni(COD)2 + 2 Al(C2H5)2(C5H7O2) + C2H4 + C2H6
The related Pt(COD)2 is prepared by a more circuitous route involving the dilithium cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
:
- Li2C8H8 + PtCl2(COD) + 3 C7H10 → [Pt(C7H10)3] + 2 LiCl + C8H8 + C8H12
- Pt(C7H10)3 + 2 COD → Pt(COD)2 + 3 C7H10
Extensive work has been reported on complexes of COD, much of which can has been described in volumes 25, 26, and 28 of Inorganic Syntheses
Inorganic Syntheses
Inorganic Syntheses is a book series which aims to publish "detailed and foolproof" procedures for the synthesis of inorganic compounds. Although this series of books are edited, they usually are referenced like a journal, without mentioning the names of the checkers or the editor. A similar...
. The platinum complex has been used in many syntheses:
- Pt(COD)2 + 3 C2H4 → Pt(C2H4)3 + 2 COD
COD complexes are useful as starting materials, one noteworthy example is the reaction:
- Ni(cod)2 + 4 CO(g)
 Ni(CO)4 + 2 COD
Ni(CO)4 + 2 COD
The product Ni(CO)4 is highly toxic, thus it is advantageous to generate it in the reaction vessel as opposed to being dispensed directly. Other low-valent metal complexes of COD include Mo(COD)(CO)4, [RuCl2(COD)]n, and Fe(COD)(CO)3. COD is an especially important in the coordination chemistry of rhodium(I) and iridium(I), examples being Crabtree's catalyst
Crabtree's catalyst
Crabtree's catalyst is the name given to a complex of iridium with 1,5-cyclooctadiene, tris-cyclohexylphosphine, and pyridine. It is a homogeneous catalyst for hydrogenation reactions, developed by Robert H. Crabtree, a professor at Yale University...
and cyclooctadiene rhodium chloride dimer. The square planar complexes [M(COD)2]+ are known (M = Rh, Ir).
(E,E)-COD
The highly strained trans-trans isomer of 1,5-cyclooctadiene is a known compound. (E,E)-COD was first synthesized by WhitesidesGeorge M. Whitesides
George M. Whitesides is an American chemist and professor of chemistry at Harvard University. He is best known for his work in the areas of NMR spectroscopy, organometallic chemistry, molecular self-assembly, soft lithography, microfabrication, microfluidics, and nanotechnology...
and Cope
Arthur C. Cope
Arthur C. Cope was a highly successful and influential organic chemist and member of the National Academy of Sciences. He is credited with the development of several important chemical reactions which bear his name including the Cope elimination and the Cope rearrangement.Cope was born on June...
in 1969 by photoisomerization of the cis compound. Another synthesis (double elimination reaction from a cyclooctane ring) was reported by Huisgen
Rolf Huisgen
Rolf Huisgen is a German chemist. He was born in Gerolstein and studied in Munich under the supervision of Heinrich Otto Wieland. After completing his Ph.D. in 1943 and his habilitation in 1947, he became professor at the University of Tübingen in 1949...
in 1987. The molecular conformation of (E,E)-COD is twisted rather than chair-like. The compound has been investigated as a click chemistry
Click chemistry
Click chemistry is a chemical philosophy introduced by K. Barry Sharpless of The Scripps Research Institute, in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together...
mediator.


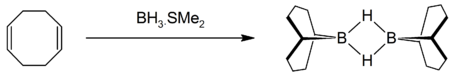
2.png)