Noble gas compound
Encyclopedia
Noble gas compounds are chemical compound
s that include an element
from Group
18 of the periodic table
, the noble gas
es.
All noble gases have full s and p outer electron shell
s (except helium
, which has no p sublevel), and so do not form chemical compound
s easily. Because of their high ionization energy
and almost zero electron affinity
, they were not expected to be reactive.
In 1933 Linus Pauling
predicted that the heavier noble gases would be able to form compounds with fluorine
and oxygen
. Specifically, he predicted the existence of krypton hexafluoride and xenon hexafluoride
(Xe
F
6), speculated that XeF8 might exist as an unstable compound, and suggested that xenic acid
would form perxenate
salts. These predictions proved quite accurate, although subsequent predictions for XeF8 indicated that it would be not only thermodynamically
unstable, but kinematically unstable
. As of 2009, XeF8 has not been made, although the octafluoroxenon(VI) anion (XeF82−) has been observed.
The heavier noble gases have more electron shells than the lighter ones. Hence, the outermost electrons experience a shielding effect
from the inner electrons that makes them more easily ionized, since they are less strongly attracted to the positively-charged nucleus
. This results in an ionization energy low enough to form stable compounds with the most electronegative
elements, fluorine and oxygen, and even with less electronegative elements such as nitrogen and carbon under certain circumstances.
(including clathrate hydrate
s). Other compounds such as coordination compounds
were observed only by spectroscopic means.
Clathrates have been used for separation of He and Ne from Ar, Kr, and Xe, and also for the transportation of Ar, Kr, and Xe. In addition,85Kr clathrate provides a safe source of beta particle
s, while 133Xe clathrate provides a useful source of gamma ray
s.
Noble gas atoms such as Kr and Xe can appear as guests in crystals of melanophlogite
.
.
ionised O2
to O2+
. As the ionisation energy of O2 to O2+ (1165 kJ mol–1) is nearly equal to the ionisation energy of Xe to Xe+ (1170 kJ mol–1), he tried the reaction of Xe with PtF6. This yielded a crystalline product xenon hexafluoroplatinate
, whose formula was proposed to be .
It was later shown that the compound is actually more complex, containing both XeFPtF6 and XeFPt2F11. This was the first real compound of any noble gas.
In September 1962, Howard Claasen reported the synthesis of a simple (two-element) noble gas compound, xenon tetrafluoride
, by subjecting xenon and fluorine to a high temperature. In November 1962, Rudolf Hoppe
of Universität Münster reported that xenon and fluorine can react to form xenon difluoride
.
In recent years, several compounds of noble gases, particularly xenon, have been prepared. Among these are the xenon fluorides (XeF2
, XeF4
, XeF6
), oxyfluorides (XeOF2, XeOF4, XeO2F2, XeO3F2, XeO2F4) and oxides (XeO3
and XeO4
). Xenon difluoride can be produced by the simple exposure of Xe and F2 gases to sunlight; while the mixing of the two gases had been tried over 50 years before in an attempt to produce a reaction, nobody had thought to simply expose the mixture to sunlight.
Xenon fluorides react with several fluorides to form fluoroxenates, such as sodium octafluoroxenate (Na+2XeF82-), and fluoroxenonium salts, such as trifluoroxenonium hexafluoroantimoniate (XeF3+SbF6-).
Recently xenon has been shown to produce a wide variety of compounds of the type XeOnX2 where n is 1,2 or 3 and X is any electronegative group, such as CF3, C(SO2CF3)3, N(SO2F)2, N(SO2CF3)2, OTeF5, O(IO2F2), etc. The range of compounds is impressive, running into the thousands and involving bonds between xenon and oxygen, nitrogen, carbon, boron and even gold, as well as perxenic acid, several halides, and complex ions; a range of compounds similar to that seen with the neighbouring element iodine
. The compound Xe2Sb2F11 contains a Xe–Xe bond, the longest element-element bond known (308.71 pm = 3.0871 Å
).
Short-lived excimer
s of Xe2 and noble gas halides such as XeCl2
are used in excimer laser
s.
, which glows with a yellow light in the solid state.
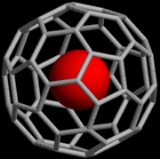
 Noble gases can also form endohedral fullerene compounds where the noble gas atom is trapped inside a fullerene
Noble gases can also form endohedral fullerene compounds where the noble gas atom is trapped inside a fullerene
molecule. In 1993, it was discovered that when C60 is exposed to a pressure of around 3 bar
of He or Ne, the complexes He@C60 and Ne@C60 are formed. Under these conditions, only about one out of every 650,000 C60 cages was doped with a helium
atom; with higher pressures (3000 bar), it is possible to achieve a yield of up to 0.1%. Endohedral complexes with argon
, krypton
and xenon
have also been obtained, as well as numerous adduct
s of He@C60.
is a valuable oxidising agent because it has no potential for introducing impurities: the xenon is simply liberated as a gas. It is rivalled only by ozone
in this respect. The perxenate
s are even more powerful oxidising agents, and the xenon fluorides are good fluorinating agents. Stable salts of xenon containing very high proportions of fluorine by weight such as perfluoroammonium heptafluoroxenon, NF4XeF7 and the related (NF4)2XeF8 have been developed as highly energetic oxidisers for use as propellants in rocketry.
Xenon-based compounds have also been used for synthesizing carbocation
s stable at room temperature in
solution.
Radioactive isotopes of krypton and xenon are difficult to store and dispose, and compounds of these elements may be more easily handled than the gaseous forms.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s that include an element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
from Group
Periodic table group
In chemistry, a group is a vertical column in the periodic table of the chemical elements. There are 18 groups in the standard periodic table, including the d-block elements, but excluding the f-block elements....
18 of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, the noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es.
History and background
It was initially believed that the noble gases could not form compounds due to their full valence shell of electrons that rendered them very chemically stable and unreactive.All noble gases have full s and p outer electron shell
Electron shell
An electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" , followed by the "2 shell" , then the "3 shell" , and so on further and further from the nucleus. The shell letters K,L,M,.....
s (except helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, which has no p sublevel), and so do not form chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s easily. Because of their high ionization energy
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
and almost zero electron affinity
Electron affinity
The Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
, they were not expected to be reactive.
In 1933 Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
predicted that the heavier noble gases would be able to form compounds with fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. Specifically, he predicted the existence of krypton hexafluoride and xenon hexafluoride
Xenon hexafluoride
Xenon hexafluoride is a noble gas compound with the formula XeF6 and the highest of the three binary fluorides of xenon, the other two being XeF2 and XeF4. All are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series...
(Xe
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
F
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
6), speculated that XeF8 might exist as an unstable compound, and suggested that xenic acid
Xenic acid
Xenic acid is a noble gas compound formed by the dissolution of xenon trioxide in water. Its chemical structure is H2XeO4. It is a very powerful oxidizing agent, and its decomposition is dangerous as it liberates a large amount of gaseous products—xenon, oxygen, and ozone.Its existence was...
would form perxenate
Perxenate
In chemistry, perxenates are salts of the yellow xenon-containing anion . This anion has octahedral molecular geometry, as determined by Raman spectroscopy, having O–Xe–O bond angles varying between 87° and 93°...
salts. These predictions proved quite accurate, although subsequent predictions for XeF8 indicated that it would be not only thermodynamically
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
unstable, but kinematically unstable
Kinematic determinacy
Kinematic determinacy is a term used in structural mechanics to describe a structure where material compatibility conditions alone can be used to calculate deflections....
. As of 2009, XeF8 has not been made, although the octafluoroxenon(VI) anion (XeF82−) has been observed.
The heavier noble gases have more electron shells than the lighter ones. Hence, the outermost electrons experience a shielding effect
Shielding effect
The shielding effect describes the decrease in attraction between an electron and the nucleus in any atom with more than one electron shell. It is also referred to as the screening effect or atomic shielding.-Cause:...
from the inner electrons that makes them more easily ionized, since they are less strongly attracted to the positively-charged nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. This results in an ionization energy low enough to form stable compounds with the most electronegative
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
elements, fluorine and oxygen, and even with less electronegative elements such as nitrogen and carbon under certain circumstances.
Pre-1962 compounds
Prior to 1962, the only isolated compounds of noble gases were clathratesClathrate compound
A clathrate, clathrate compound or cage compound is a chemical substance consisting of a lattice of one type of molecule trapping and containing a second type of molecule...
(including clathrate hydrate
Hydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
s). Other compounds such as coordination compounds
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
were observed only by spectroscopic means.
Clathrates
Clathrates (also known as cage compounds) are compounds of noble gases in which they are trapped within cavities of crystal lattices of certain organic and inorganic substances. The essential condition for their formation is that the guest (noble gas) atoms should be of appropriate size to fit in the cavities of the host crystal lattice. For instance, Ar, Kr, and Xe can form clathrates with β-quinol, but He and Ne cannot fit because they are too small.Clathrates have been used for separation of He and Ne from Ar, Kr, and Xe, and also for the transportation of Ar, Kr, and Xe. In addition,85Kr clathrate provides a safe source of beta particle
Beta particle
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
s, while 133Xe clathrate provides a useful source of gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s.
Noble gas atoms such as Kr and Xe can appear as guests in crystals of melanophlogite
Melanophlogite
Melanophlogite is a rare silicate mineral and a polymorph of silica . It has a zeolite-like porous structure which results in relatively low and not well-defined values of its density and refractive index. Melanophlogite often overgrows crystals of sulfur or calcite and typically contains a few...
.
Coordination compounds
Coordination compounds such as Ar·BF3 have been postulated to exist at low temperatures, but have never been confirmed. Also, compounds such as WHe2 and HgHe2 were reported to have been formed by electron bombardment, but recent research has shown that these are probably the result of He being adsorbed on the surface of the metal; therefore, these compounds cannot truly be considered chemical compounds.Hydrates
Hydrates are formed by compressing the noble gases in water. It is believed that the water molecule, a strong dipole, induces a weak dipole in the noble gas atoms, resulting in dipole-dipole interaction. Heavier atoms are more influenced than smaller ones, hence Xe·6H2O is the most stable hydrate. The existence of these compounds has, however, been disputed in recent years.Helium compounds
Although there is some theoretical evidence for a few metastable helium compounds which may be stable at very low temperatures and extreme pressures, none are confirmed by experiments.Krypton compounds
Krypton is able to react with fluorine to form KrF2Krypton difluoride
Krypton difluoride, KrF2, was the first compound of krypton discovered. It is a volatile, colourless solid. The structure of the KrF2 molecule is linear, with Kr−F distances of 188.9 pm...
.
Xenon compounds
The first published report, in June 1962, of a noble gas compound was by Neil Bartlett, who noticed that the highly oxidising compound platinum hexafluoridePlatinum hexafluoride
Platinum hexafluoride is the chemical compound with the formula PtF6. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state...
ionised O2
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to O2+
Dioxygenyl
The dioxygenyl ion, O2+, is a rarely encountered oxycation in which both oxygen atoms have a formal oxidation state of +½. It is formally derived from oxygen by the removal of an electron:...
. As the ionisation energy of O2 to O2+ (1165 kJ mol–1) is nearly equal to the ionisation energy of Xe to Xe+ (1170 kJ mol–1), he tried the reaction of Xe with PtF6. This yielded a crystalline product xenon hexafluoroplatinate
Xenon hexafluoroplatinate
Xenon hexafluoroplatinate is the name of the product of the reaction of platinum hexafluoride and xenon, in an experiment that proved the chemical reactivity of the noble gases...
, whose formula was proposed to be .
It was later shown that the compound is actually more complex, containing both XeFPtF6 and XeFPt2F11. This was the first real compound of any noble gas.
In September 1962, Howard Claasen reported the synthesis of a simple (two-element) noble gas compound, xenon tetrafluoride
Xenon tetrafluoride
Xenon tetrafluoride is a chemical compound with chemical formula . It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine, , according to the chemical equation:...
, by subjecting xenon and fluorine to a high temperature. In November 1962, Rudolf Hoppe
Rudolf Hoppe
Rudolf Hoppe , a German chemist, discovered the first covalent noble gas compounds.-Academic career:...
of Universität Münster reported that xenon and fluorine can react to form xenon difluoride
Xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture sensitive. It decomposes on contact with light or water vapour. Xenon difluoride is a dense, white crystalline solid. It...
.
In recent years, several compounds of noble gases, particularly xenon, have been prepared. Among these are the xenon fluorides (XeF2
Xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture sensitive. It decomposes on contact with light or water vapour. Xenon difluoride is a dense, white crystalline solid. It...
, XeF4
Xenon tetrafluoride
Xenon tetrafluoride is a chemical compound with chemical formula . It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine, , according to the chemical equation:...
, XeF6
Xenon hexafluoride
Xenon hexafluoride is a noble gas compound with the formula XeF6 and the highest of the three binary fluorides of xenon, the other two being XeF2 and XeF4. All are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series...
), oxyfluorides (XeOF2, XeOF4, XeO2F2, XeO3F2, XeO2F4) and oxides (XeO3
Xenon trioxide
Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly , accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials...
and XeO4
Xenon tetroxide
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas...
). Xenon difluoride can be produced by the simple exposure of Xe and F2 gases to sunlight; while the mixing of the two gases had been tried over 50 years before in an attempt to produce a reaction, nobody had thought to simply expose the mixture to sunlight.
Xenon fluorides react with several fluorides to form fluoroxenates, such as sodium octafluoroxenate (Na+2XeF82-), and fluoroxenonium salts, such as trifluoroxenonium hexafluoroantimoniate (XeF3+SbF6-).
Recently xenon has been shown to produce a wide variety of compounds of the type XeOnX2 where n is 1,2 or 3 and X is any electronegative group, such as CF3, C(SO2CF3)3, N(SO2F)2, N(SO2CF3)2, OTeF5, O(IO2F2), etc. The range of compounds is impressive, running into the thousands and involving bonds between xenon and oxygen, nitrogen, carbon, boron and even gold, as well as perxenic acid, several halides, and complex ions; a range of compounds similar to that seen with the neighbouring element iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
. The compound Xe2Sb2F11 contains a Xe–Xe bond, the longest element-element bond known (308.71 pm = 3.0871 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
).
Short-lived excimer
Excimer
An excimer is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which is in an electronic excited state. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state. The lifetime of an excimer is...
s of Xe2 and noble gas halides such as XeCl2
Xenon dichloride
Xenon dichloride is the only chloride of xenon which can be produced by chemical reactions. Its formula is XeCl2. The compound can be prepared by using microwave discharge technique towards the mixture of xenon and chlorine, and it can be isolated from condensate trap...
are used in excimer laser
Excimer laser
An excimer laser is a form of ultraviolet laser which is commonly used in the production of microelectronic devices , eye surgery, and micromachining....
s.
Radon compounds
Radon reacts with fluorine to form RnF2Radon fluoride
Radon difluoride is a compound of radon, a noble gas. Radon reacts readily with fluorine to form a solid compound, but this decomposes on attempted vaporization and its exact composition is uncertain. Calculations suggest that it may be ionic. The usefulness of radon compounds is limited because...
, which glows with a yellow light in the solid state.
Fullerene compounds

Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
molecule. In 1993, it was discovered that when C60 is exposed to a pressure of around 3 bar
Bar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
of He or Ne, the complexes He@C60 and Ne@C60 are formed. Under these conditions, only about one out of every 650,000 C60 cages was doped with a helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
atom; with higher pressures (3000 bar), it is possible to achieve a yield of up to 0.1%. Endohedral complexes with argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
, krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
and xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
have also been obtained, as well as numerous adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
s of He@C60.
Applications
Most applications of noble gas compounds are either as oxidising agents or as a means to store noble gases in a dense form. Xenic acidXenic acid
Xenic acid is a noble gas compound formed by the dissolution of xenon trioxide in water. Its chemical structure is H2XeO4. It is a very powerful oxidizing agent, and its decomposition is dangerous as it liberates a large amount of gaseous products—xenon, oxygen, and ozone.Its existence was...
is a valuable oxidising agent because it has no potential for introducing impurities: the xenon is simply liberated as a gas. It is rivalled only by ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
in this respect. The perxenate
Perxenate
In chemistry, perxenates are salts of the yellow xenon-containing anion . This anion has octahedral molecular geometry, as determined by Raman spectroscopy, having O–Xe–O bond angles varying between 87° and 93°...
s are even more powerful oxidising agents, and the xenon fluorides are good fluorinating agents. Stable salts of xenon containing very high proportions of fluorine by weight such as perfluoroammonium heptafluoroxenon, NF4XeF7 and the related (NF4)2XeF8 have been developed as highly energetic oxidisers for use as propellants in rocketry.
Xenon-based compounds have also been used for synthesizing carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s stable at room temperature in
Sulfuryl chloride fluoride
Sulfuryl chloride fluoride is the chemical compound with the formula SO2ClF. It is employed as a solvent for highly oxidizing compounds.The laboratory-scale synthesis begins with the preparation of potassium fluorosulfite:This salt is then chlorinated to give sulfuryl chloride fluorideFurther...
solution.
Radioactive isotopes of krypton and xenon are difficult to store and dispose, and compounds of these elements may be more easily handled than the gaseous forms.
Resources
- http://www.chemsoc.org/exemplarchem/entries/2001/robson/raregascompounds.htm
- http://www.webelements.com/webelements/elements/

