
Natural oil polyols
Encyclopedia
Natural oil polyols, also known as NOPs or biopolyols, are polyols derived from vegetable oils by several different techniques. The primary use for these materials is in the production of polyurethanes. Most NOPs qualify as Biobased Product
s, as defined by the United States Secretary of Agriculture in the Farm Security and Rural Investment Act of 2002.
NOPs all have similar sources and applications, but the materials themselves can be quite different, depending on how they are made. All are clear liquids, ranging from colorless to medium yellow. Their viscosity
is also variable and is usually a function of the molecular weight and the average number of hydroxyl
groups per molecule (higher mw and higher hydroxyl content both giving higher viscosity.) Odor is a significant property which is different from NOP to NOP. Most NOPs are still quite similar chemically to their parent vegetable oils and as such are prone to becoming rancid
. This involves autoxidation
of fatty acid chains containing carbon-carbon double bonds and ultimately the formation of odoriferous, low molecular weight aldehydes, ketones and carboxylic acids. Odor is undesirable in the NOPs themselves, but more importantly, in the materials made from them.
There are a limited number of naturally occurring vegetable oils (triglycerides) which contain the unreacted hydroxyl groups that account for both the name and important reactivity of these polyols. Castor oil
is the only commercially-available natural oil polyol that is produced directly from a plant source: all other NOPs require chemical modification of the oils directly available from plants.
The hope is that using renewable resources as feedstocks for chemical processes will reduce the environmental footprint by reducing the demand on non-renewable fossil fuels currently used in the chemical industry and reduce the overall production of carbon dioxide
, the most notable greenhouse gas
. One NOP producer, Cargill, estimates that its BiOH(TM)polyol manufacturing process produces 36% less global warming
emissions (carbon dioxide), a 61% reduction in non-renewable energy use (burning fossil fuels), and a 23% reduction in the total energy demand, all relative to polyols produced from petrochemical
s..
is ricinoleic acid
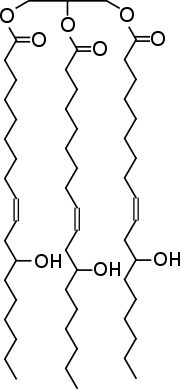
, which has a hydroxyl
group on C-12 and a carbon-carbon double bond. The structure below shows the major component of castor oil which is composed of the tri-ester of rincinoleic acid and glycerin:
 Other vegetal oils - such as soy bean oil, peanut oil
Other vegetal oils - such as soy bean oil, peanut oil
, and canola oil - contain carbon-carbon double bonds, but no hydroxyl groups. There are several processes used to introduce hydroxyl groups onto the carbon chain of the fatty acids, and most of these involve oxidation
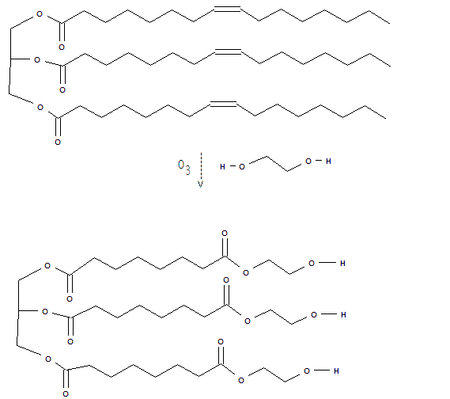
of the C-C double bond. Treatment of the vegetal oils with ozone
cleaves the double bond, and esters or alcohols can be made, depending on the conditions used to process the ozonolysis
product. The example below shows the reaction of triolein
with ozone and ethylene glycol
.
 Air oxidation, (autoxidation
Air oxidation, (autoxidation
), the chemistry involved in the "drying" of drying oils, gives increased molecular weight and introduces hydroxyl groups. The radical reactions
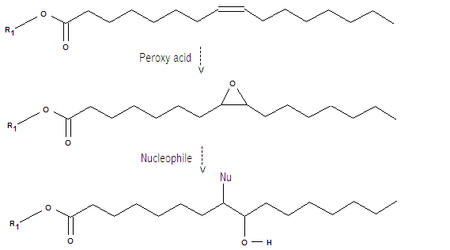
involved in autoxidation can produce a complex mixture of crosslinked and oxidized triglycerides. Treatment of vegetable oils with peroxy acid
s gives epoxides which can be reacted with nucleophiles to give hydroxyl groups. This can be done as a one-step process. Note that in the example shown below only one of the three fatty acid chains is drawn fully, the other part of the molecule is represented by "R1" and the nucleophile is unspecified. Earlier examples also include acid catalyzed ring opening of epoxidized soybean oil
to make oleochemical polyols for polyurethane foams and acid catalyzed ring opening of soy fatty acid methyl esters with multifunctional polyols to form new polyols for casting resins .
 Triglycerides of unsaturated (containing carbon-carbon double bonds) fatty acids or methyl esters of these acids, can be treated with carbon monoxide
Triglycerides of unsaturated (containing carbon-carbon double bonds) fatty acids or methyl esters of these acids, can be treated with carbon monoxide
and hydrogen
in the presence of a metal catalyst to add a -CHO (formyl) groups to the chain (hydroformylation
reaction) followed by hydrogenation
to give the needed hydroxyl groups. In this case R1 can be the rest of the triglyceride, or a smaller group such as methyl (in which case the substrate would be similar to biodiesel
). If R=Me then additional reactions like transesterification
are needed to build up a polyol.

Castor oil by itself has been used in making a variety of polyurethane products, ranging from coatings to foams, and the use of castor oil derivatives continues to be an area of active development. Castor oil derivatized with propylene oxide
makes polyurethane foam for mattresses and yet another new derivative is used in coatings
Apart from castor oil, which is a relatively expensive vegetable oil and is not produced domestically in many industrialized countries, the use of polyols derived from vegetable oils to make polyurethane products began attracting attention beginning around 2004. The rising costs of petrochemical feedstocks and an enhanced public desire for environmentally friendly
green
products have created a demand for these materials.. One of the most vocal supporters of these polyurethanes made using natural oil polyols is the Ford Motor Company
, which first debuted polyurethane foam made using soy oil in the seats of its 2008 Ford Mustang
.. Ford has since placed soy foam seating in all its North American vehicle platforms. The interest of automakers is responsible for much of the work being done on the use of NOPs in polyurethane products for use in cars, for example is seats, and headrests, armrests, soundproofing, and even body panels..
NOPs are also finding use in polyurethane slab foam used to make conventional mattresses as well as memory foam
mattresses.
One of the first uses for NOPs (other than castor oil) was to make spray-on polyurethane foam insulation for buildings.
Biobased Product
Biobased product, was defined by the United States Secretary of Agriculture in the Farm Security and Rural Investment Act of 2002 as follows, "The term ‘‘biobased product’’ means a product determined by the Secretary to be a commercial or industrial product that is composed, in whole or in...
s, as defined by the United States Secretary of Agriculture in the Farm Security and Rural Investment Act of 2002.
NOPs all have similar sources and applications, but the materials themselves can be quite different, depending on how they are made. All are clear liquids, ranging from colorless to medium yellow. Their viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
is also variable and is usually a function of the molecular weight and the average number of hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups per molecule (higher mw and higher hydroxyl content both giving higher viscosity.) Odor is a significant property which is different from NOP to NOP. Most NOPs are still quite similar chemically to their parent vegetable oils and as such are prone to becoming rancid
Rancidification
Rancidification is the chemical decomposition of fats, oils and other lipids . When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable . In processed meats, these flavors are collectively known as "warmed over flavor"...
. This involves autoxidation
Autoxidation
Autoxidation is any oxidation that occurs in open air or in presence of oxygen and/or UV radiation and forms peroxides and hydroperoxides. A classic example of autoxidation is that of simple ethers like diethyl ether, whose peroxides can be dangerously explosive. It can be considered to be a slow,...
of fatty acid chains containing carbon-carbon double bonds and ultimately the formation of odoriferous, low molecular weight aldehydes, ketones and carboxylic acids. Odor is undesirable in the NOPs themselves, but more importantly, in the materials made from them.
There are a limited number of naturally occurring vegetable oils (triglycerides) which contain the unreacted hydroxyl groups that account for both the name and important reactivity of these polyols. Castor oil
Castor oil
Castor oil is a vegetable oil obtained from the castor bean . Castor oil is a colorless to very pale yellow liquid with mild or no odor or taste. Its boiling point is and its density is 961 kg/m3...
is the only commercially-available natural oil polyol that is produced directly from a plant source: all other NOPs require chemical modification of the oils directly available from plants.
The hope is that using renewable resources as feedstocks for chemical processes will reduce the environmental footprint by reducing the demand on non-renewable fossil fuels currently used in the chemical industry and reduce the overall production of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, the most notable greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
. One NOP producer, Cargill, estimates that its BiOH(TM)polyol manufacturing process produces 36% less global warming
Global warming
Global warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
emissions (carbon dioxide), a 61% reduction in non-renewable energy use (burning fossil fuels), and a 23% reduction in the total energy demand, all relative to polyols produced from petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
s..
Sources of natural oil polyols
Ninety percent of the fatty acids that make up castor oilCastor oil
Castor oil is a vegetable oil obtained from the castor bean . Castor oil is a colorless to very pale yellow liquid with mild or no odor or taste. Its boiling point is and its density is 961 kg/m3...
is ricinoleic acid
Ricinoleic acid
Ricinoleic acid is an unsaturated omega-9 fatty acid that naturally occurs in mature Castor plant seeds or in sclerotium of ergot . About 90% of the fatty acid content in castor oil is the triglyceride formed from ricinoleic acid...
, which has a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group on C-12 and a carbon-carbon double bond. The structure below shows the major component of castor oil which is composed of the tri-ester of rincinoleic acid and glycerin:

Peanut oil
Peanut oil is an organic material oil derived from peanuts, noted to have the aroma and taste of its parent legume....
, and canola oil - contain carbon-carbon double bonds, but no hydroxyl groups. There are several processes used to introduce hydroxyl groups onto the carbon chain of the fatty acids, and most of these involve oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of the C-C double bond. Treatment of the vegetal oils with ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
cleaves the double bond, and esters or alcohols can be made, depending on the conditions used to process the ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
product. The example below shows the reaction of triolein
Triolein
Triolein is a triglyceride and unsaturated fat formed from oleic acid. Triolein is used in Semipermeable membrane devices to collect organic compounds in form of a passive sampling device in water analysis. the collecting compound triolein is placed in a tube and set up in a cage like container...
with ozone and ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
.

Autoxidation
Autoxidation is any oxidation that occurs in open air or in presence of oxygen and/or UV radiation and forms peroxides and hydroperoxides. A classic example of autoxidation is that of simple ethers like diethyl ether, whose peroxides can be dangerously explosive. It can be considered to be a slow,...
), the chemistry involved in the "drying" of drying oils, gives increased molecular weight and introduces hydroxyl groups. The radical reactions
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
involved in autoxidation can produce a complex mixture of crosslinked and oxidized triglycerides. Treatment of vegetable oils with peroxy acid
Peroxy acid
A peroxy acid is an acid which contains an acidic -OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the organic derivatives of carboxylic acids...
s gives epoxides which can be reacted with nucleophiles to give hydroxyl groups. This can be done as a one-step process. Note that in the example shown below only one of the three fatty acid chains is drawn fully, the other part of the molecule is represented by "R1" and the nucleophile is unspecified. Earlier examples also include acid catalyzed ring opening of epoxidized soybean oil
Epoxidized soybean oil
Epoxidized soybean oil, better known by its acronym, ESBO, is a plasticizer used in polyvinyl chloride plastics. It serves as a plasticizer and as a scavenger for hydrochloric acid liberated from PVC when the PVC undergoes heat treatment....
to make oleochemical polyols for polyurethane foams and acid catalyzed ring opening of soy fatty acid methyl esters with multifunctional polyols to form new polyols for casting resins .

Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
in the presence of a metal catalyst to add a -CHO (formyl) groups to the chain (hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
reaction) followed by hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
to give the needed hydroxyl groups. In this case R1 can be the rest of the triglyceride, or a smaller group such as methyl (in which case the substrate would be similar to biodiesel
Biodiesel
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl esters. Biodiesel is typically made by chemically reacting lipids with an alcohol....
). If R=Me then additional reactions like transesterification
Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R′ of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst...
are needed to build up a polyol.

Uses
Castor oil has found numerous applications, many of them due to the presence of the hydroxyl group that allows chemical derivatization of the oil or modifies the properties of castor oil relative to vegetable oils which do not have the hydroxyl group. Castor oil undergoes most of the reactions that alcohols do, but the most industrially important one is reaction with diisocyanates to make polyurethanes.Castor oil by itself has been used in making a variety of polyurethane products, ranging from coatings to foams, and the use of castor oil derivatives continues to be an area of active development. Castor oil derivatized with propylene oxide
Propylene oxide
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics...
makes polyurethane foam for mattresses and yet another new derivative is used in coatings
Apart from castor oil, which is a relatively expensive vegetable oil and is not produced domestically in many industrialized countries, the use of polyols derived from vegetable oils to make polyurethane products began attracting attention beginning around 2004. The rising costs of petrochemical feedstocks and an enhanced public desire for environmentally friendly
Environmentally friendly
Environmentally friendly are terms used to refer to goods and services, laws, guidelines and policies claimed to inflict minimal or no harm on the environment....
green
Green chemistry
Green chemistry, also called sustainable chemistry, is a philosophy of chemical research and engineering that encourages the design of products and processes that minimize the use and generation of hazardous substances...
products have created a demand for these materials.. One of the most vocal supporters of these polyurethanes made using natural oil polyols is the Ford Motor Company
Ford Motor Company
Ford Motor Company is an American multinational automaker based in Dearborn, Michigan, a suburb of Detroit. The automaker was founded by Henry Ford and incorporated on June 16, 1903. In addition to the Ford and Lincoln brands, Ford also owns a small stake in Mazda in Japan and Aston Martin in the UK...
, which first debuted polyurethane foam made using soy oil in the seats of its 2008 Ford Mustang
Ford Mustang
The Ford Mustang is an automobile manufactured by the Ford Motor Company. It was initially based on the second generation North American Ford Falcon, a compact car. Introduced early on April 17, 1964, as a "1964½" model, the 1965 Mustang was the automaker's most successful launch since the Model A...
.. Ford has since placed soy foam seating in all its North American vehicle platforms. The interest of automakers is responsible for much of the work being done on the use of NOPs in polyurethane products for use in cars, for example is seats, and headrests, armrests, soundproofing, and even body panels..
NOPs are also finding use in polyurethane slab foam used to make conventional mattresses as well as memory foam
Memory foam
Memory foam is polyurethane with additional chemicals increasing its viscosity and density. It is often referred to as "visco-elastic" polyurethane foam, or low-resilience polyurethane foam . Higher-density memory foam softens in reaction to body heat, allowing it to mold to a warm body in a few...
mattresses.
One of the first uses for NOPs (other than castor oil) was to make spray-on polyurethane foam insulation for buildings.

